47132
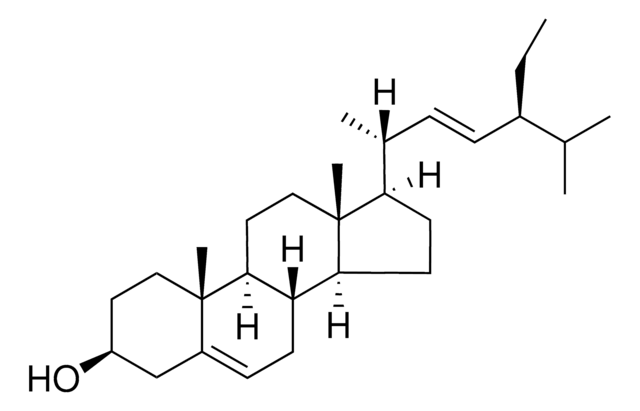
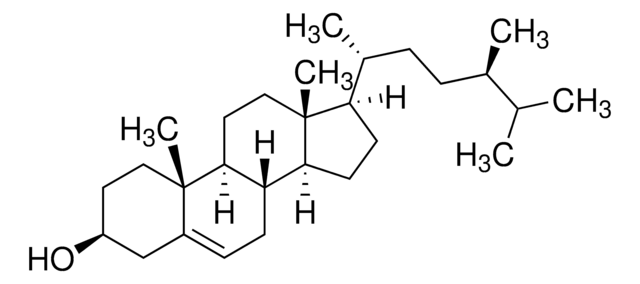
Stigmasterol
certified reference material, 10 mg/mL in chloroform
Sinonimo/i:
3β-Hydroxy-24-ethyl-5,22-cholestadiene, 5,22-Stigmastadien-3β-ol, Stigmasterin
About This Item
Prodotti consigliati
Grado
certified reference material
TraceCERT®
Nome Commerciale
TraceCERT®
Saggio
95% (chromatography)
Forma fisica
liquid
CdA
current certificate can be downloaded
Confezionamento
ampule of 1 mL
Concentrazione
10 mg/mL in chloroform
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Punto di fusione
165-167 °C (lit.)
applicazioni
food and beverages
Formato
single component solution
Gruppo funzionale
hydroxyl
Temperatura di conservazione
2-30°C
room temp
Stringa SMILE
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)C3=CC2)([H])CC[C@@]4(C)[C@@]1([H])CC[C@]4([H])[C@]([H])(C)/C=C/[C@@H](CC)C(C)C
InChI
1S/C29H48O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h8-10,19-21,23-27,30H,7,11-18H2,1-6H3/b9-8+/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1
HCXVJBMSMIARIN-PHZDYDNGSA-N
Informazioni sul gene
human ... POLA1(5422) , TOP2A(7153)
rat ... Polb(29240)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Altre note
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Organi bersaglio
Central nervous system, Liver,Kidney
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
Separation of Cholesterol; Brassicasterol; Campesterol; Stigmasterol; β-Sitosterol
Separation of Cholesterol; Brassicasterol; Campesterol; Stigmasterol; β-Sitosterol
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.