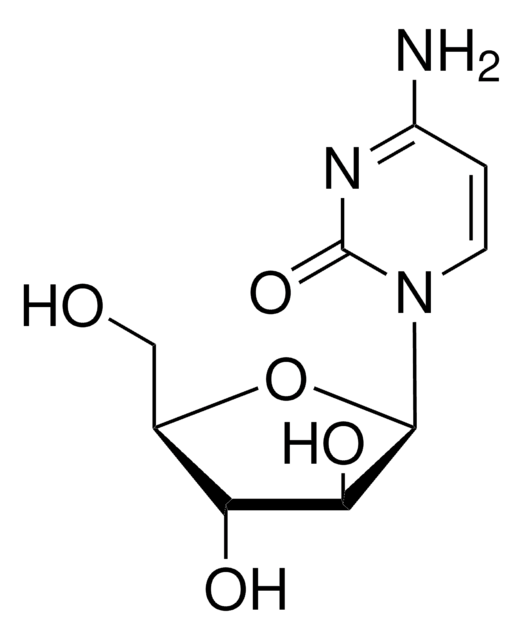
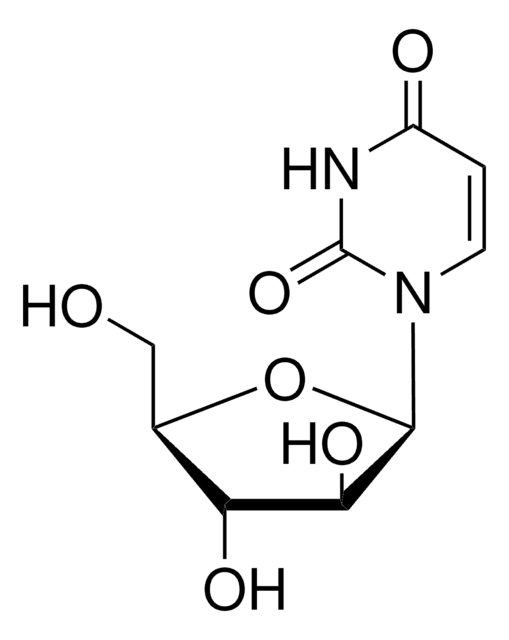
T3766
Thymine 1-β-D-arabinofuranoside
≥99%
Sinonimo/i:
araT, 1-β-D-Arabinofuranosylthymine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H14N2O6
Numero CAS:
Peso molecolare:
258.23
Numero CE:
Numero MDL:
Codice UNSPSC:
41106305
ID PubChem:
NACRES:
NA.51
Prodotti consigliati
Livello qualitativo
Saggio
≥99%
Stato
powder
Attività ottica
[α]20/D 90°, c = 5 mg/mL in water
Solubilità
0.5 M HCl: 20 mg/mL, clear, colorless
Temperatura di conservazione
2-8°C
Stringa SMILE
CC1=CN(C2OC(CO)C(O)C2O)C(=O)NC1=O
InChI
1S/C10H14N2O6/c1-4-2-12(10(17)11-8(4)16)9-7(15)6(14)5(3-13)18-9/h2,5-7,9,13-15H,3H2,1H3,(H,11,16,17)
DWRXFEITVBNRMK-UHFFFAOYSA-N
Applicazioni
1-β-D-Arabinofuranosylthymine (araT):
- maybe used as a substrate and inhibitor to study the specificity and kinetics of thymidine kinase(s), such as herpes simplex virus type 1 and 2 thymidine kinases, the HSV1-tk and, HSV2-tk gene products.
- is used to study the specificity and kinetics of various mitochondrial nucleoside kinases.
- has been used as an antiviral agent to study its efficacy on Cryptosporidium parvum in vitro.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
N Solaroli et al.
Antiviral research, 79(2), 128-132 (2008-05-06)
The thymidine kinases from feline herpesvirus (FHV TK) and canine herpesvirus (CHV TK) were cloned and characterized. The two proteins are closely sequence-related to each other and also to the herpes simplex virus type 1 thymidine kinase (HSV-1 TK). Although
E S Arnér et al.
Biochemical and biophysical research communications, 188(2), 712-718 (1992-10-30)
Human cells salvage pyrimidine deoxyribonucleosides via 5'-phosphorylation which is also the route of activation of many chemotherapeutically used nucleoside analogs. Key enzymes in this metabolism are the cytosolic thymidine kinase (TK1), the mitochondrial thymidine kinase (TK2) and the cytosolic deoxycytidine
Ada Bertoli et al.
The FEBS journal, 272(15), 3918-3928 (2005-07-28)
The multisubstrate nucleoside kinase of Drosophila melanogaster (Dm-dNK) can be expressed in human solid tumor cells and its unique enzymatic properties makes this enzyme a suicide gene candidate. In the present study, Dm-dNK was stably expressed in the CCRF-CEM and
Ana-Isabel Hernandez et al.
Journal of medicinal chemistry, 49(26), 7766-7773 (2006-12-22)
Novel N1-substituted thymine derivatives related to 1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine have been synthesized and evaluated against thymidine kinase-2 (TK-2) and related nucleoside kinases [i.e., Drosophila melanogaster deoxynucleoside kinase (Dm-dNK) and herpes simplex virus type 1 thymidine kinase (HSV-1 TK)]. The thymine base has
J Balzarinia et al.
Biochemical pharmacology, 61(6), 727-732 (2001-03-27)
Introduction of a bulky lipophilic acyl entity at the 2'-OH position of both 1-beta-D-arabinofuranosylthymine (araT) and (E)-5-(2-bromovinyl)-1-beta-D-arabinofuranosyluracil (BVaraU), consistently resulted in a marked ( approximately 10-fold) increase in the inhibitory activity of these new arabinosyl nucleoside analogues for the mitochondrial
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.