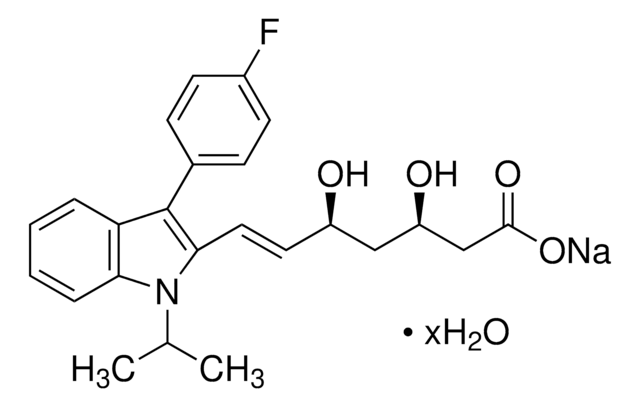
F8682
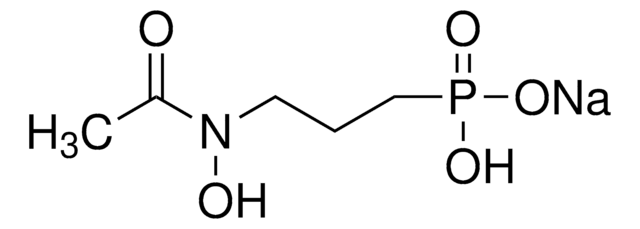
Fosmidomycin sodium salt hydrate
≥95% (NMR)
Sinonimo/i:
(3-(Formylhydroxyamino)propyl)phosphonic acid sodium salt, (3-(N-Hydroxyformamido)propyl)phosphonic acid sodium salt, FR 31564
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H10NO5P · xNa+ · yH2O
Numero CAS:
Peso molecolare:
183.10 (anhydrous free acid basis)
Numero MDL:
Codice UNSPSC:
12352200
NACRES:
NA.77
Prodotti consigliati
Saggio
≥95% (NMR)
Stato
powder
Condizioni di stoccaggio
desiccated
Colore
white to beige
Solubilità
H2O: 20 mg/mL, clear
Temperatura di conservazione
−20°C
Stringa SMILE
[P](=O)(O)(O)CCCN(O)C=O
InChI
1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10)
GJXWDTUCERCKIX-UHFFFAOYSA-N
Categorie correlate
Applicazioni
Fosmidomycin sodium salt hydrate has been used as an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in a study to determine monotropin carvacrol biosynthesis in Satureja khuzistanica plant.
Azioni biochim/fisiol
Fosmidomycin is an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) (MEP synthase): an antimalarial compound.
Fosmidomycin is an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) (MEP synthase): an antimalarial compound. 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) is an enzyme involved in the first step in the nonmevalonate pathway for isoprenoid biosynthesis in Gram-negative, Gram-positive bacteria, plants, and the parasite causing the most virulent form of malaria, Plasmodium falciparum (Mammals produce isoprenoids via the mevalonate pathway).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Karin Brücher et al.
Journal of medicinal chemistry, 55(14), 6566-6575 (2012-06-27)
Specific inhibition of enzymes of the non-mevalonate pathway is a promising strategy for the development of novel antiplasmodial drugs. α-Aryl-substituted β-oxa isosteres of fosmidomycin with a reverse orientation of the hydroxamic acid group were synthesized and evaluated for their inhibitory
Christoph T Behrendt et al.
Journal of medicinal chemistry, 54(19), 6796-6802 (2011-08-27)
Reverse hydroxamate-based inhibitors of IspC, a key enzyme of the non-mevalonate pathway of isoprenoid biosynthesis and a validated antimalarial target, were synthesized and biologically evaluated. The binding mode of one derivative in complex with EcIspC and a divalent metal ion
Emily R Jackson et al.
Current topics in medicinal chemistry, 12(7), 706-728 (2012-01-31)
Isoprene biosynthesis is an essential component of metabolism. Two pathways are known for the production of five-carbon (isoprene) intermediates: the mevalonate and nonmevalonate pathways. As many pathogenic organisms rely exclusively on the nonmevalonate pathway (NMP) for isoprenoids and humans do
Robert R Junker et al.
Journal of chemical ecology, 37(12), 1323-1331 (2011-12-14)
In their natural environment, plants are synchronously confronted with mutualists and antagonists, and thus benefit from signals that contain messages for both functional groups of interaction partners. Floral scents are complex blends of volatiles of different chemical classes, including benzenoids
Catherine Zinglé et al.
Bioorganic & medicinal chemistry letters, 22(21), 6563-6567 (2012-10-03)
Fosmidomycin derivatives in which the hydroxamic acid group has been replaced by several bidentate chelators as potential hydroxamic alternatives were prepared and tested against the DXR from Escherichia coli. These results illustrate the predominant role of the hydroxamate functional group
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.