E9906
Anti-EDEM2 (N-terminal) antibody produced in rabbit
~1.0 mg/mL, affinity isolated antibody, buffered aqueous solution
Sinonimo/i:
Anti-ER degradation enhancer, mannosidase alpha-like 2
About This Item
Prodotti consigliati
Origine biologica
rabbit
Coniugato
unconjugated
Forma dell’anticorpo
affinity isolated antibody
Tipo di anticorpo
primary antibodies
Clone
polyclonal
Forma fisica
buffered aqueous solution
PM
antigen ~70 kDa
Reattività contro le specie
human, rat (predicted), mouse
Concentrazione
~1.0 mg/mL
tecniche
indirect immunofluorescence: 5-10 μg/mL using mouse 3T3 cells
western blot: 0.5-1 μg/mL using whole extracts of HEK-293T cells expressing recombinant human EDEM2
N° accesso UniProt
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
modifica post-traduzionali bersaglio
unmodified
Informazioni sul gene
human ... EDEM2(55741)
mouse ... Edem2(108687)
rat ... Edem2(296304)
Descrizione generale
Immunogeno
Applicazioni
Azioni biochim/fisiol
Stato fisico
Esclusione di responsabilità
Non trovi il prodotto giusto?
Prova il nostro Motore di ricerca dei prodotti.
Prodotti correlati
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.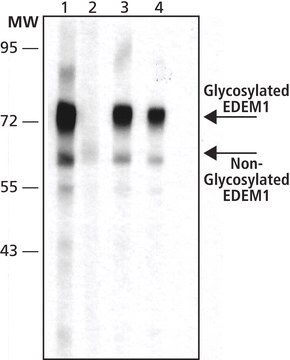
![(±)-(E)-4-Ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide ≥98%](/deepweb/assets/sigmaaldrich/product/structures/404/859/0c6eb80d-2c3c-4bf1-98e8-1b319b597b12/640/0c6eb80d-2c3c-4bf1-98e8-1b319b597b12.png)