C9879
Bovine Collagen Type I
from bovine achilles tendon, powder, suitable for substrate for collagenase
Sinonimo/i:
Collagen powder
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Nome del prodotto
Collagen from bovine achilles tendon, powder, suitable for substrate for collagenase
Origine biologica
bovine Achilles tendon
Stato
powder
tecniche
ELISA: suitable
activity assay: suitable
Compatibilità
suitable for substrate for collagenase
N° accesso UniProt
Temperatura di conservazione
2-8°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
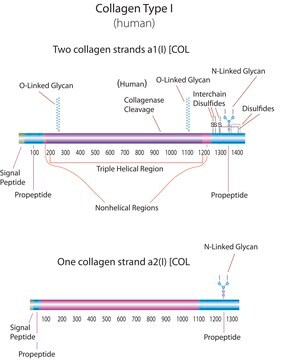
Collagen is a widely expressed protein in the body. There are 20 different types of collagen in human tissue. Type I collagen is the abundant bone protein. It constitutes ~90% of bone organic matter.
Collagen is classified into a number of structurally and genetically distinct types. We use the nomenclature proposed by Bornstein and Traub. Do not confuse Sigma type designations with recognized collagen classification types.
Collagen terminology using the Bornstein and Traub designation originates from the reference; Bornstein, P. and Traub, W. The Proteins, (1979) 4, 411-605
Applicazioni
Bovine achilles tendon collagen was used in a study of plant extracts as inhibitors of MMP-collagenases, the putative active agents in the breakdown of cartilage in osteoarthritis and rheumatoid arthritis.
Collagen from bovine achilles tendon is suitable for use in:
- the detection of collagenase activity
- as a reference sample in the thermal analysis study of human bone using differential scanning calorimetry, thermogravimetry, gas chromatography and Fourier transform infrared spectroscopy
- as a substrate for developing a simple assay for determining collagen degradation in vitro
- a study to examine the binding activity of the integral glycoprotein dipeptidyl peptidase IV to insoluble type I collagen by solid-phase enzyme-linked immunosorbent assay
Azioni biochim/fisiol
Collagen from bovine Achilles tendon is a naturally occurring protein in the form of elongated fibrils. It may be used in studies of the fibrocartilaginous zone, which the collagen must first pass through before inserting into the calcaneus. It may also be used in studies of growth factor effects on collagen content and cross-linking during Achilles tendon healing.
Collagen is an insoluble fibrous protein that is part of the extracellular matrix and the connective tissue. It is responsible for the ability of the tissues to withstand stretching. The long precursors called procollagens are synthesized and assembled in the ER, secreted into the extracellular space and processed to form collagen fibres. The different types of collagen are composed of molecules containing three polypeptide chains arranged in a triple helical conformation varying slightly in the amino acid sequence. The primary structure is a repeating motif with glycine in every third position preceded frequently with a proline or 4-hydroxyproline residue.
Nota sulla preparazione
Prepared by the method of Einbinder, J. and Schubert, M., J. Biol. Chem., 188, 335 (1951).
Ricostituzione
This product is an insoluble collagen preparation. It is insoluble in water, aqueous buffers, dilute acid, and organic solvents. For use as a substrate in collagenase assays, this collagen can be prepared as a suspension in 50 mM TES buffer, pH 7.4 with 0.36 mM calcium chloride.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
J M Nagmoti et al.
Indian journal of medical microbiology, 26(1), 65-67 (2008-01-30)
Anaerobic gram-negative bacteria (AGNB) produce enzymes that play a significant role in the development of disease. We tested 50 AGNB isolates, 25 each from clinically diseased and healthy human sites for in vitro production of caseinase, collagenase, etc. Majority of
Yumi Kumagai et al.
Infection and immunity, 73(5), 2655-2664 (2005-04-23)
Porphyromonas gingivalis is a pathogen associated with adult periodontitis. It produces dipeptidyl aminopeptidase IV (DPPIV), which may act as a virulence factor by contributing to the degradation of connective tissue. We investigated the molecular mechanism by which DPPIV contributes to
E K Steffen et al.
Journal of clinical microbiology, 14(2), 153-156 (1981-08-01)
Thirty-three strains of anaerobic bacteria isolated from human clinical specimens were examined for the presence of heparinase, hyaluronidase, chondroitin sulfatase, gelatinase, collagenase, fibrinolysin, lecithinase, and lipase activities. Pronounced heparinase activity was limited to species of the genus Bacteroides. A number
S Bjelland et al.
Archives of dermatological research, 280(1), 50-53 (1988-01-01)
A simple assay measuring degradation of human epidermal keratin and bovine tendon collagen is presented. Insoluble protein substrate (30 mg) was incubated with 1 ml buffer and enzyme sample for 1 h at 37 degrees C, following addition of 1
M L Tanzer
Science (New York, N.Y.), 180(4086), 561-566 (1973-05-11)
The formation of collagen cross-links is attributable to the presence of two aldehyde-containing amino acids which react with other amino acids in collagen to generate difunctional, trifunctional, and tetrafunctional cross-links. A necessary prerequisite for the development of these cross-links is
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.