A9934
Aminopeptidase I from Streptomyces griseus
lyophilized powder, ≥200 units/mg protein
Sinonimo/i:
Leucine Aminopeptidase IV
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Stato
lyophilized powder
Livello qualitativo
Attività specifica
≥200 units/mg protein
PM
21 kDa by gel filtration
33 kDa by SDS-PAGE
Composizione
Protein, 40-60% Lowry
Temperatura di conservazione
−20°C
Descrizione generale
Aminopeptidase I from Streptomyces griseus is a thermostable enzyme with Glu131 and Tyr246 as key active site residues.
Applicazioni
Aminopeptidase I from Streptomyces griseus has been used:
- to test the biochar exposure effect on the enzyme activity
- in circular dichroism (CD) spectroscopy studies
- as a positive control in p-nitroanilide degradation assay
Azioni biochim/fisiol
Aminopeptidase I from S. griseus has a fairly broad specificity, being able to remove the N-terminal residue of most proteins, except where the penultimate residue is an imino acid. It contains two Zn2+ binding sites. Aminopeptidase I from S. griseus is inhibited by 1,10-phenanthroline and is activated six-fold by Ca2+, which also stabilizes it against heat inactivation. This monomeric zinc metalloprotein has an isoelectric point (pI) of 5.4.
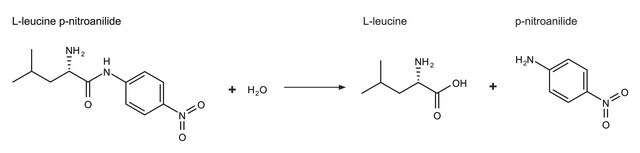
Aminopeptidase I may also be used as a reagent in the assay of endoprotease activities with a synthetic substrate in a two-stage assay. In the first stage, the endoprotease cleaves a peptide, such as Z-Y-X-Leu-p-nitroanilide, with the X, Y, and Z residues being chosen according to the specificity of the endoprotease.
Confezionamento
Package size based on protein content.
Definizione di unità
One unit will hydrolyze 1.0 μmole of L-leucine-p-nitroanilide to L-leucine and p-nitroaniline per min at pH 8.0, 25 °C and 3.0 mM substrate concentration.
Stato fisico
Contains calcium acetate
Nota sulla preparazione
Reconstitute in 20 mM tricine, pH 8.0, with 0.05% bovine serum albumin. Dilute the enzyme with the reconstitution buffer to 0.15-0.3 U/mL for a working concentration. Solutions should be prepared fresh prior to use.
Altre note
Endopeptidase contaminant: Not more than: 0.01 U/mg protein (as μmole tyrosine equivalent per min released from casein.)
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
[Autophagy related genes in yeast, S. cerevisiae].
Yoshinori Ohsumi
Tanpakushitsu kakusan koso. Protein, nucleic acid, enzyme, 51(10 Suppl), 1453-1456 (2006-08-23)
Indig, F.E., et al.
Febs Letters, 225, 237-237 (1989)
Taras Y Nazarko et al.
Autophagy, 1(1), 37-45 (2006-07-29)
Yarrowia lipolytica was recently introduced as a new model organism to study peroxisome degradation in yeasts. Transfer of Y. lipolytica cells from oleate/ethylamine to glucose/ammonium chloride medium leads to selective macroautophagy of peroxisomes. To decipher the molecular mechanisms of macropexophagy
A Spungin et al.
European journal of biochemistry, 183(2), 471-477 (1989-08-01)
A heat-stable aminopeptidase with an N-terminal Ala-Pro-Asp-Ile-Pro-Leu sequence has been purified from Streptomyces griseus by heat treatment followed by gel-exclusion and anion-exchange chromatographic procedures. The enzyme is a monomeric zinc metalloenzyme showing an apparent molecular mass of 33 kDa by
Congcong He et al.
Molecular biology of the cell, 19(12), 5506-5516 (2008-10-03)
Autophagy is the degradation of a cell's own components within lysosomes (or the analogous yeast vacuole), and its malfunction contributes to a variety of human diseases. Atg9 is the sole integral membrane protein required in formation of the initial sequestering
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.