47568
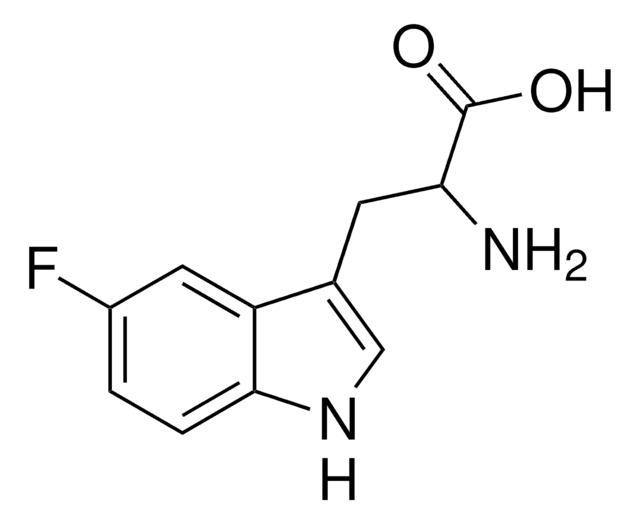
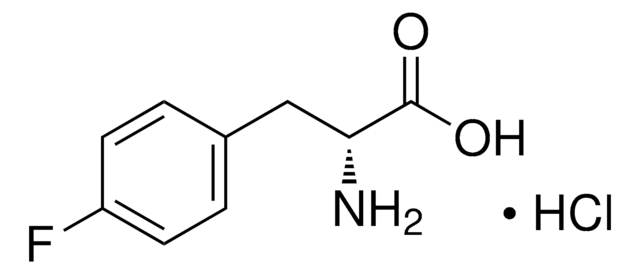
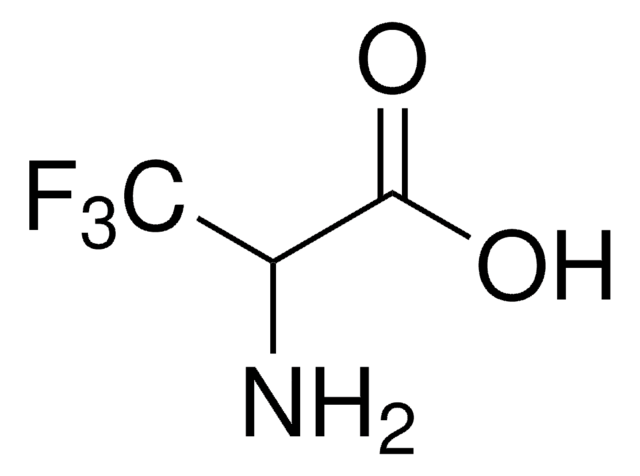
5-Fluoro-L-tryptophan
≥98.0% (HPLC)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H11FN2O2
Numero CAS:
Peso molecolare:
222.22
Beilstein:
5052680
Numero MDL:
Codice UNSPSC:
12352202
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.77
Prodotti consigliati
Saggio
≥98.0% (HPLC)
Stato
powder
Purezza ottica
enantiomeric ratio: ≥99.5:0.5 (HPLC)
Punto di fusione
270-280 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
N[C@@H](Cc1c[nH]c2ccc(F)cc12)C(O)=O
InChI
1S/C11H11FN2O2/c12-7-1-2-10-8(4-7)6(5-14-10)3-9(13)11(15)16/h1-2,4-5,9,14H,3,13H2,(H,15,16)/t9-/m0/s1
INPQIVHQSQUEAJ-VIFPVBQESA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Exogenous 5-fluoro-Trp is incorporated into proteins in normal protein synthesis. Since 19F is a useful reporter group, this provides a method for studying enzyme mechanisms by NMR.
Azioni biochim/fisiol
5-Fluoro-Trp is nonspecifically cytotoxic. It is believed this is due to malfunctioning enzymes that have had replacements of Trp residues by 5-fluoro-Trp. However, at least one case is known where 5-fluoro-Trp substitution leads to significantly greater catalytic activity.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
G S Rule et al.
Biochemistry, 26(2), 549-556 (1987-01-27)
In this study we demonstrate the potential of combining fluorine-19 nuclear magnetic resonance (NMR) spectroscopy with molecular genetics. We are using the membrane-bound enzyme D-lactate dehydrogenase of Escherichia coli as a model system to characterize interactions between proteins and lipids.
Dereje Abate Negatu et al.
mBio, 10(2) (2019-03-28)
Indole propionic acid (IPA), produced by the gut microbiota, is active against Mycobacterium tuberculosisin vitro and in vivo However, its mechanism of action is unknown. IPA is the deamination product of tryptophan (Trp) and thus a close structural analog of
E W Miles et al.
Biochemistry, 25(15), 4240-4249 (1986-07-29)
We are exploring the active site and the mechanism of the pyridoxal phosphate dependent reactions of the bacterial tryptophan synthase alpha 2 beta 2 complex by use of substrate analogues and of reaction intermediate analogues. Fluorine-19 nuclear magnetic resonance studies
Warintra Pitsawong et al.
eLife, 7 (2018-06-15)
Protein kinases are major drug targets, but the development of highly-selective inhibitors has been challenging due to the similarity of their active sites. The observation of distinct structural states of the fully-conserved Asp-Phe-Gly (DFG) loop has put the concept of
S Rozovsky et al.
Journal of molecular biology, 310(1), 271-280 (2001-06-23)
Product release is partially rate determining in the isomerization reaction catalyzed by Triosephosphate Isomerase, the conversion of dihydroxyacetone phosphate to D-glyceraldehyde 3-phosphate, probably because an active-site loop movement is necessary to free the product from confinement in the active-site. The
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.