17794
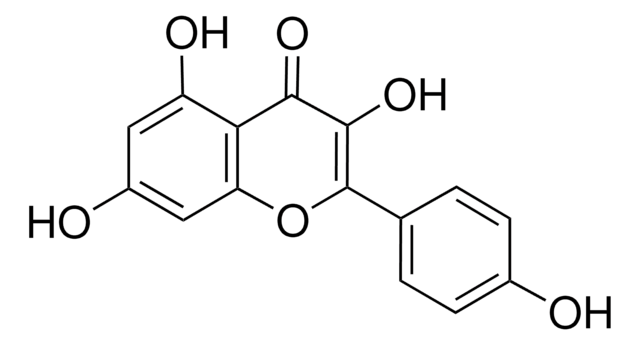
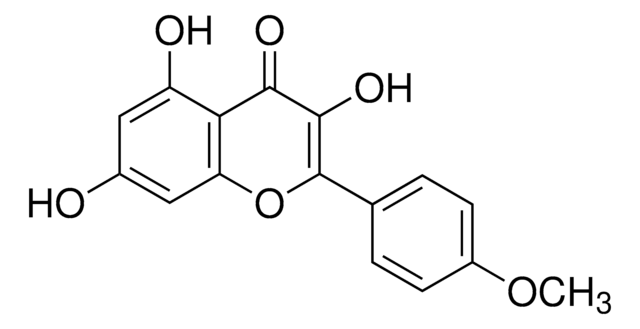
Isorhamnetin
≥95.0% (HPLC)
Sinonimo/i:
3′-Methoxy-3,4′,5,7-tetrahydroxyflavone, 3′-Methylquercetin, 3,4′,5,7-Tetrahydroxy 3′-methoxyflavone, Isorhamnetol, Quercetin 3′-methyl ether
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0% (HPLC)
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Stringa SMILE
COc1cc(ccc1O)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C16H12O7/c1-22-11-4-7(2-3-9(11)18)16-15(21)14(20)13-10(19)5-8(17)6-12(13)23-16/h2-6,17-19,21H,1H3
IZQSVPBOUDKVDZ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as a neuroprotective agent to test its effect on scopolamine-induced cortico-hippocampal learning and memory deficiency in mice
- as a reference standard in quadrupole time-of-flight mass spectrometry (QTOF MS) to analyze and quantify the phenolic compounds present in honey extract
- as a reference standard to determine the phenolic profile of Artemisia species using Reversed-phase high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry (RP-HPLC-DAD-ESI-TQ-MS/MS)
Azioni biochim/fisiol
Confezionamento
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.