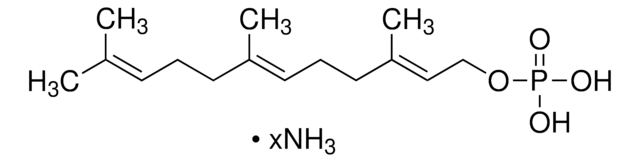
12436
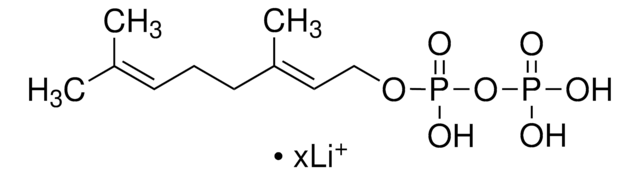
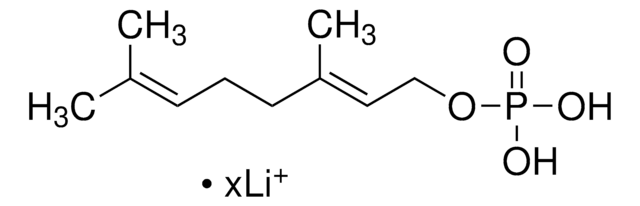
Neryl pyrophosphate lithium salt
≥95.0% (TLC)
Sinonimo/i:
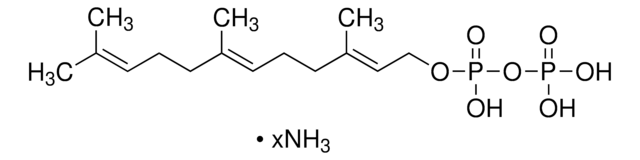
(Z)-3,7-Dimethyl-2,6-octadien-1-yl pyrophosphate lithium salt, Neryl diphosphate lithium salt
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H20O7P2 · xLi+
Numero CAS:
Peso molecolare:
314.21 (free acid basis)
Numero MDL:
Codice UNSPSC:
12352107
NACRES:
NA.25
Prodotti consigliati
Applicazioni
Neryl pyrophosphate, the cis isomer of geranyl pyrophosphate, may be used to characterize and study the kinetics of enzymes such as 1,8-cineole synthase, farnesyl pyrophosphate synthase, pinene cyclase and geranyl pyrophosphate:sabinene hydrate cyclase.
Azioni biochim/fisiol
Metabolite, substrate for monoterpene synthase.
Confezionamento
Bottomless glass bottle. Contents are inside inserted fused cone.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
R Croteau et al.
Archives of biochemistry and biophysics, 309(1), 184-192 (1994-02-15)
Geranyl pyrophosphate: 1,8-cineole cyclase (cineole synthase) catalyzes the conversion of geranyl pyrophosphate to the symmetrical monoterpene ether 1,8-cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane) by a process thought to involve the initial isomerization of the substrate to the tertiary allylic isomer, linalyl pyrophosphate, and cyclization
R Croteau et al.
The Journal of biological chemistry, 264(26), 15309-15315 (1989-09-15)
(+)-Pinene cyclase from sage (Salvia officinalis) catalyzes the isomerization and cyclization of geranyl pyrophosphate to (+)-alpha-pinene and (+)-camphene, and to lesser amounts of (+)-limonene, myrcene, and terpinolene, whereas (-)-pinene cyclase from this tissue catalyzes the conversion of the acyclic precursor
D R Light et al.
The Journal of biological chemistry, 264(31), 18598-18607 (1989-11-05)
A prenyltransferase purified from the commercial rubber tree, Hevea brasiliensis, that elongates existing cis-polyisoprene rubber molecules also catalyzes the formation of all trans-farnesyl pyrophosphate (t,t-FPP) from dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP). In assays of the latter activity trans-geranyl
T W Hallahan et al.
Archives of biochemistry and biophysics, 264(2), 618-631 (1988-08-01)
A soluble enzyme preparation from the leaves of sweet marjoram (Majorana hortensis Moench) catalyzes the divalent cation-dependent cyclization of [1-3H]geranyl pyrophosphate to the bicyclic monoterpene alcohols (+)-[6-3H]cis- and (+)-[6-3H]-transsabinene hydrate, providing labeling patterns consistent with current mechanistic considerations. No free
Old substrates for new enzymes of terpenoid biosynthesis.
Jörg Bohlmann et al.
Proceedings of the National Academy of Sciences of the United States of America, 106(26), 10402-10403 (2009-06-26)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.