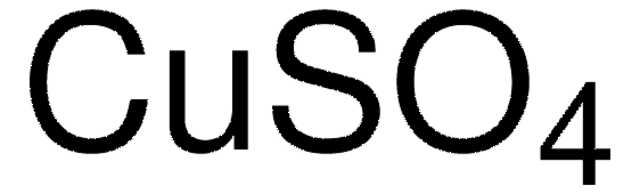12852
Copper(II) sulfate
puriss., meets analytical specification of Ph. Eur., BP, USP, anhydrous, 99-100.5% (based on anhydrous substance)
Sinonimo/i:
Cupric sulfate
About This Item
Prodotti consigliati
Tensione di vapore
7.3 mmHg ( 25 °C)
Livello qualitativo
Grado
puriss.
Saggio
99-100.5% (based on anhydrous substance)
Forma fisica
solid
Qualità
meets analytical specification of Ph. Eur., BP, USP
Impurezze
residual solvents, complies
Perdita
≤1% loss on drying, 250 °C
pH
3.5-4.5 (20 °C, 50 g/L)
Punto di fusione
200 °C (dec.) (lit.)
Densità
3.603 g/mL at 25 °C (lit.)
Anioni in tracce
chloride (Cl-): ≤100 mg/kg
Cationi in tracce
Ca: ≤50 mg/kg
Fe: ≤30 mg/kg
K: ≤100 mg/kg
Mg: ≤100 mg/kg
Na: ≤200 mg/kg
Ni: ≤50 mg/kg
Pb: ≤80 mg/kg
Stringa SMILE
[Cu++].[O-]S([O-])(=O)=O
InChI
1S/Cu.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2
ARUVKPQLZAKDPS-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
It can find applications in agriculture as a nutrient for plants. It can also serve as a precursor in the production of foliar bactericides and fungicides.
Applicazioni
- As a catalyst for the acetylation of alcohols and phenols under solvent-free conditions.
- To compose the electrolyte for the electrodeposition of Cu-Zn-Sn precursors, required for the preparation of Cu2ZnSnS4 (CZTS) thin films.
- As a Lewis acid catalyst for the dehydration of alcohols.5
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.