HLA002
Jurkat HLA Panel Cell Lines
Sinonimo/i:
CAR-T, Checkpoint Inhibitors, Immuno-oncology, Immunotherapy, Oncology
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
41106514
Prodotti consigliati
Origine biologica
human (T Lymphocyte (blood))
Modalità di accrescimento
suspension
Condizioni di spedizione
dry ice
Temperatura di conservazione
−196°C
Descrizione generale
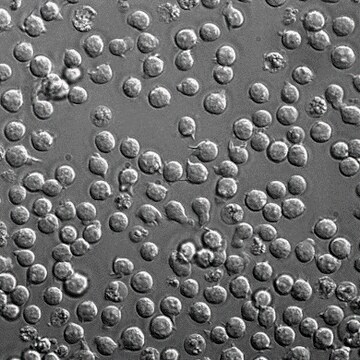
The STR profile of these cell lines match that of its parental cell line - ECACC catalog number 88042803.
This product is a panel of eleven (11) genetically modified cell lines targeting MHC Class I HLA molecules in a Jurkat T lymphocyte parental cell line background. The full HLA panel consists of a Beta-2 microglobulin (β2M) knockout cell line and an additional ten (10) monoallelic HLA expression cell lines.
CompoZr® Zinc Finger Nuclease (ZFN) technology was used to generate the β2M knockout cell line, which does not express endogenous cell surface MHC Class I HLA molecules (HLA-A or HLA-B) as measured by fluorescence-activated cell sorting (FACS).
MISSION® lentivirus was used to generate the ten (10) monoallelic HLA cell lines, which express individual HLA-A or HLA-B subtypes on the cell surface via a β2M:HLA fusion protein as described in Nature Biotechnology 35, 765-772, (2017) and as demonstrated via FACS analysis.
This product is a panel of eleven (11) genetically modified cell lines targeting MHC Class I HLA molecules in a Jurkat T lymphocyte parental cell line background. The full HLA panel consists of a Beta-2 microglobulin (β2M) knockout cell line and an additional ten (10) monoallelic HLA expression cell lines.
CompoZr® Zinc Finger Nuclease (ZFN) technology was used to generate the β2M knockout cell line, which does not express endogenous cell surface MHC Class I HLA molecules (HLA-A or HLA-B) as measured by fluorescence-activated cell sorting (FACS).
MISSION® lentivirus was used to generate the ten (10) monoallelic HLA cell lines, which express individual HLA-A or HLA-B subtypes on the cell surface via a β2M:HLA fusion protein as described in Nature Biotechnology 35, 765-772, (2017) and as demonstrated via FACS analysis.
Descrizione della linea cellulare
Derived from Jurkat FHCRC. An IL-2 producing cell line, derived by incubating the cells at 41°C for 48 hours followed by a limiting dilution cloning over macrophages.
Caratteristiche e vantaggi
Unlike wild type Jurkat cells, which express multiple HLA subtypes, the Jurkat HLA panel cell lines express individual MHC Class I HLA subtypes. These monoallelic HLA cell lines can be used to study peptide binding and presentation in the context of individual HLA subtypes. The panel can also be used to develop cell-based assays to evaluate candidate cancer immunotherapies and identify tumor-specific neoantigens.
Componenti
HLA002A-1VL: Jurkat B2M KO Cells
HLA002B-1VL: Jurkat HLA-A*02:01 Cells
HLA002C-1VL: Jurkat HLA-A*01:01 Cells
HLA002D-1VL: Jurkat HLA-A*03:01 Cells
HLA002E-1VL: Jurkat HLA-A*11:01 Cells
HLA002F-1VL: Jurkat HLA-A*24:02 Cells
HLA002G-1VL: Jurkat HLA-B*15:10 Cells
HLA002H-1VL: Jurkat HLA-B*07:02 Cells
HLA002I-1VL: Jurkat HLA-B*08:01 Cells
HLA002J-1VL: Jurkat HLA-B*35:01 Cells
HLA002K-1VL: Jurkat HLA-B*40:01 Cells
HLA002B-1VL: Jurkat HLA-A*02:01 Cells
HLA002C-1VL: Jurkat HLA-A*01:01 Cells
HLA002D-1VL: Jurkat HLA-A*03:01 Cells
HLA002E-1VL: Jurkat HLA-A*11:01 Cells
HLA002F-1VL: Jurkat HLA-A*24:02 Cells
HLA002G-1VL: Jurkat HLA-B*15:10 Cells
HLA002H-1VL: Jurkat HLA-B*07:02 Cells
HLA002I-1VL: Jurkat HLA-B*08:01 Cells
HLA002J-1VL: Jurkat HLA-B*35:01 Cells
HLA002K-1VL: Jurkat HLA-B*40:01 Cells
Qualità
Tested for Mycoplasma, bacterial and fungal content, post-freeze viability, and short terminal repeat (STR) analysis for cell line identification.
Note legali
CompoZr is a registered trademark of Merck KGaA, Darmstadt, Germany
MISSION is a registered trademark of Merck KGaA, Darmstadt, Germany
Esclusione di responsabilità
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Codice della classe di stoccaggio
10 - Combustible liquids
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.