42604
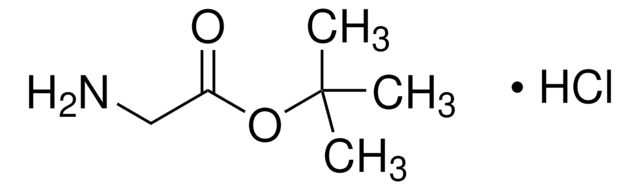
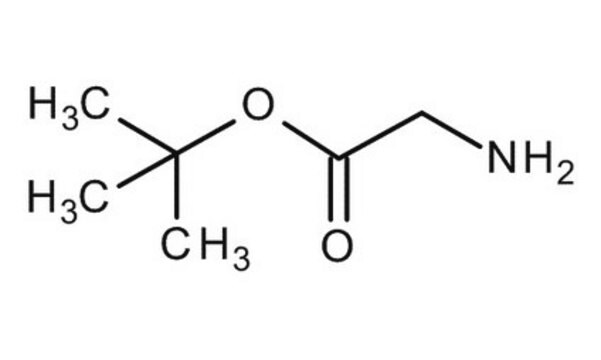
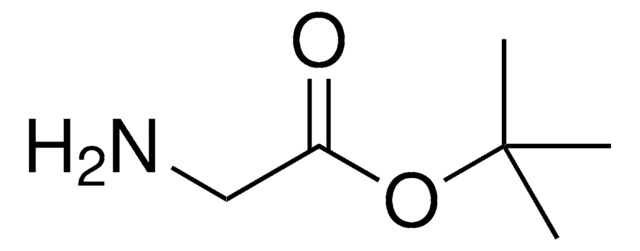
Glycine tert-butyl ester hydrochloride
puriss., ≥99.0% (AT)
Sinonimo/i:
tert-Butyl aminoacetate hydrochloride
About This Item
Prodotti consigliati
Grado
puriss.
Livello qualitativo
Saggio
≥99.0% (AT)
Forma fisica
solid
Impiego in reazioni chimiche
reaction type: solution phase peptide synthesis
Punto di fusione
141-143 °C (lit.)
Solubilità
H2O: 0.1 g/mL, clear, colorless
applicazioni
peptide synthesis
Stringa SMILE
Cl.CC(C)(C)OC(=O)CN
InChI
1S/C6H13NO2.ClH/c1-6(2,3)9-5(8)4-7;/h4,7H2,1-3H3;1H
OSWULUXZFOQIRU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.