414247
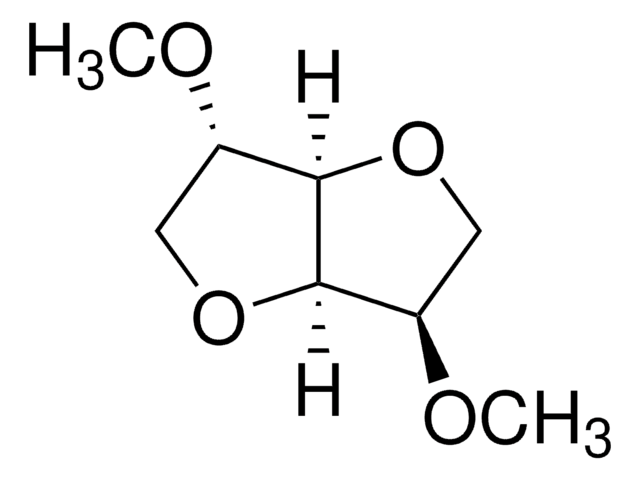
2-metiltetraidrofurano
BioRenewable, anhydrous, ≥99.0%, contains 250 ppm BHT as stabilizer
Sinonimo/i:
2-MeTHF, Tetraidro-2-metilfurano, Tetraidrosilvano
About This Item
Prodotti consigliati
Grado
anhydrous
Livello qualitativo
Saggio
≥99.0%
Stato
liquid
contiene
250 ppm BHT as stabilizer
Limite di esplosione
0.34-6.3 %
Caratteristiche più verdi
Safer Solvents and Auxiliaries
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
<0.002% water
<0.005% water (100 mL pkg)
Residuo dopo evaporazione
<0.0003%
Indice di rifrazione
n20/D 1.406 (lit.)
P. ebollizione
78-80 °C (lit.)
Punto di fusione
-136 °C
Densità
0.86 g/mL at 25 °C (lit.)
Categoria alternativa più verde
Stringa SMILE
CC1CCCO1
InChI
1S/C5H10O/c1-5-3-2-4-6-5/h5H,2-4H2,1H3
JWUJQDFVADABEY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2-Methyltetrahydrofuran (2-MTHF), a 2-methyl substituted tetrahdrofuran, is a biomass derived solvent. It is a potential greener solvent alternative for organic synthesis. It shows resistance to reduction by lithium making it a promising candidate as electrolytes in lithium batteries. Its polarity and Lewis base strength is intermediate between tetrahydrofuran (THF) and diethyl ether. The ring opening reaction of 2-MTHF has been studied using acid chloride and iodide to form secondary chlorides and primary iodides respectively. On long term storage, tetrahydrofuran forms organic peroxides. This process can be suppressed by adding butylated hydroxytoluene (BHT) as a stabilizer. BHT removes the free radicals required for the peroxide formation.
Applicazioni
It may be used as an alternative solvent to:
- DMSO (dimethyl sulfoxide) or MTBE (methyl tertiary butyl ether) in the C-C bond forming reactions catalyzed by lyase enzyme.
- THF in the reaction between Grignard reagents and carbonyl compounds.
- Methylene chloride in some biphase reactions.
Organic Solar Cells
2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry
Caratteristiche e vantaggi
Confezionamento
Altre note
Note legali
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2
Rischi supp
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
14.0 °F - closed cup
Punto d’infiammabilità (°C)
-10.0 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.