03-4991
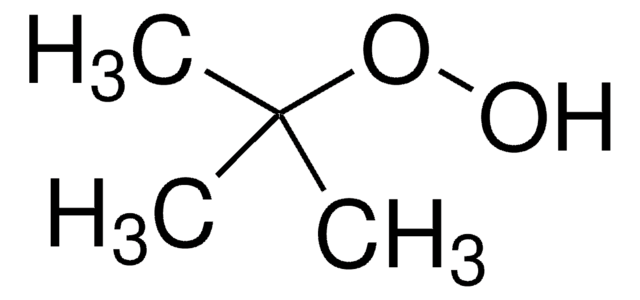
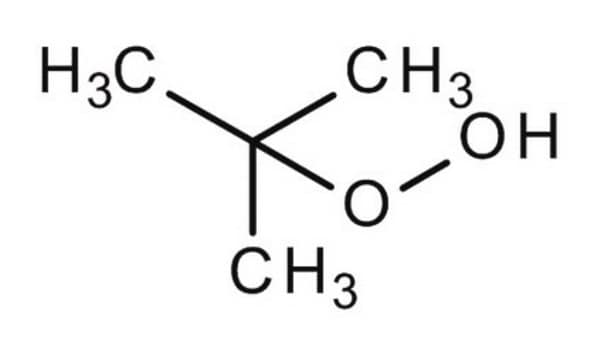
tert-Butyl hydroperoxide solution
CP
Sinonimo/i:
1,1-Dimethylethyl hydroperoxide, 2-Hydroperoxy-2-methylpropane, TBHP
About This Item
Prodotti consigliati
Grado
CP
Forma fisica
liquid
Disponibilità
available only in Japan
Concentrazione
69 wt. % in H2O
pH
4.3
Stringa SMILE
CC(C)(C)OO
InChI
1S/C4H10O2/c1-4(2,3)6-5/h5H,1-3H3
CIHOLLKRGTVIJN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Muta. 2 - Org. Perox. F - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
5.2 - Organic peroxides and self-reacting hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
107.6 °F - closed cup
Punto d’infiammabilità (°C)
42 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.