T-058
Tapentadol hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
Sinonimo/i:
Tapentadol hydrochloride solution
About This Item
Prodotti consigliati
Grado
certified reference material
Forma fisica
liquid
Caratteristiche
Snap-N-Spike®/Snap-N-Shoot®
Confezionamento
ampule of 1 mL
Produttore/marchio commerciale
Cerilliant®
drug control
Narcotic Licence Schedule A (Switzerland); estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
Concentrazione
1.0 mg/mL in methanol (as free base)
tecniche
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
applicazioni
forensics and toxicology
Formato
single component solution
Temperatura di conservazione
−20°C
Stringa SMILE
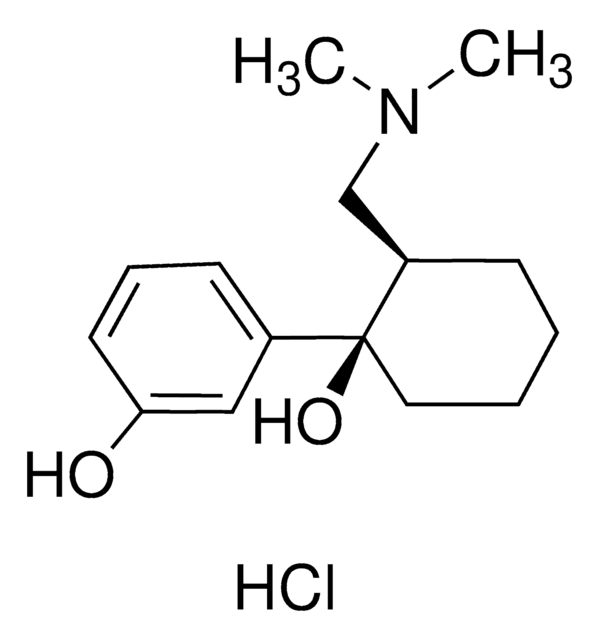
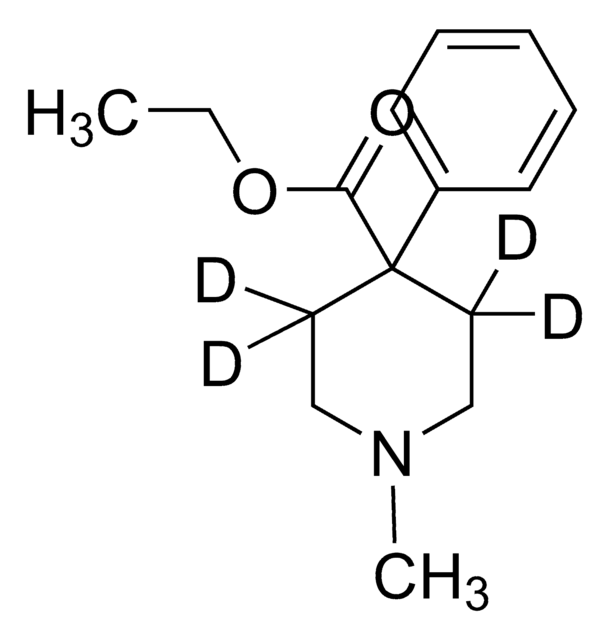
Cl.CC[C@H]([C@@H](C)CN(C)C)c1cccc(O)c1
InChI
1S/C14H23NO.ClH/c1-5-14(11(2)10-15(3)4)12-7-6-8-13(16)9-12;/h6-9,11,14,16H,5,10H2,1-4H3;1H/t11-,14+;/m0./s1
ZELFLGGRLLOERW-YECZQDJWSA-N
Informazioni sul gene
human ... OPRM1(4988) , SLC6A2(6530)
Categorie correlate
Descrizione generale
Applicazioni
- Extended-Release Formulation for Chronic Pain: Extended-release formulations of Tapentadol hydrochloride are developed to provide sustained pain relief for chronic conditions. This formulation reduces the frequency of dosing and enhances patient compliance, offering consistent pain management (Faria et al., 2017).
- Pharmaceutical Quality Control and Research: High-purity Tapentadol hydrochloride solutions are essential in pharmaceutical research and quality control. These solutions are used for the calibration of analytical instruments and ensuring the consistency and safety of pharmaceutical products (Fejos et al., 2014).
- Transdermal Delivery Systems: Innovative research has developed PEGylated ultra-deformable transferosomes for the transdermal delivery of Tapentadol, improving its bioavailability and analgesic activity. This method offers a non-invasive alternative for pain management (Deng et al., 2022).
- Innovative Pain Management Solutions: Research on new delivery methods, such as intranasal administration using chitosan nanoparticles, aims to enhance the therapeutic potential and patient compliance of Tapentadol hydrochloride solution for pain management (Javia & Thakkar, 2017).
Note legali
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organi bersaglio
Eyes,Central nervous system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
49.5 °F - closed cup
Punto d’infiammabilità (°C)
9.7 °C - closed cup
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Protocolli
-THC solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.