850400C
Avanti
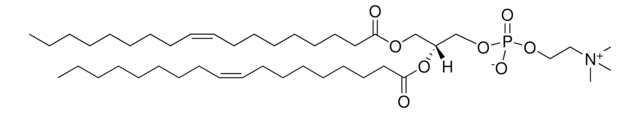
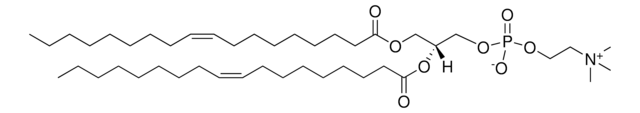
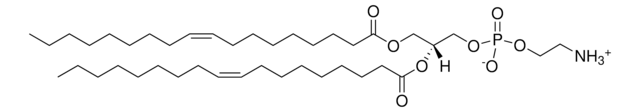
22:6 (Cis) PC
1,2-didocosahexaenoyl-sn-glycero-3-phosphocholine, chloroform
Sinonimo/i:
1,2-di-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycero-3-phosphocholine; PC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z))
About This Item
Prodotti consigliati
Saggio
>99% (TLC)
Stato
liquid
Confezionamento
pkg of 1 × 2.5 mL (850400C-25mg)
pkg of 5 × 4 mL (850400C-500mg)
Produttore/marchio commerciale
Avanti Research™ - A Croda Brand 850400C
Concentrazione
10 mg/mL (850400C-25mg)
25 mg/mL (850400C-500mg)
Tipo di lipide
cardiolipins
phospholipids
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
InChI
1S/C52H80NO8P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-34-36-38-40-42-44-51(54)58-48-50(49-60-62(56,57)59-47-46-53(3,4)5)61-52(55)45-43-41-39-37-35-33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h8-11,14-17,20-23,26-29,32-35,38-41,50H,6-7,12-13,18-19,24-25,30-31,36-37,42-49H2,1-5H3/b10-8-,11-9-,16-14-,17-15-,22-20-,23-21-,28-26-,29-27-,34-32-,35-33-,40-38-,41-39-/t50-/m1/s1
XLKQWAMTMYIQMG-SVUPRYTISA-N
Descrizione generale
Applicazioni
- in liposomes, to study its effect on membrane vesiculation by dynamin and endophilin
- in multi-lamellar vesicles (MLVs) to analyze its effect on the biophysical properties of lipid membranes and on its interaction with a fragment of the Aβ peptide
- in lipid bilayers to study the influence of cholesterol on lateral segregation of saturated and unsaturated phospholipids
Confezionamento
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Organi bersaglio
Central nervous system, Liver,Kidney
Codice della classe di stoccaggio
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
does not flash
Punto d’infiammabilità (°C)
does not flash
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.