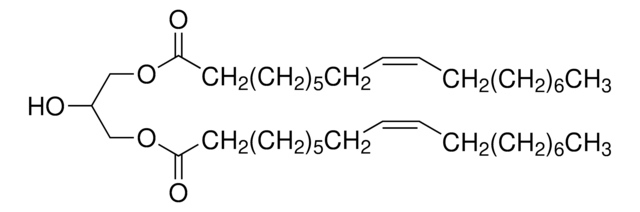800819C
Avanti
18:0-22:6 DG
1-stearoyl-2-docosahexaenoyl-sn-glycerol, chloroform
Sinonimo/i:
1-octadecanoyl-2-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycerol; DG(18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0)
About This Item
Prodotti consigliati
Saggio
>99% (TLC)
Forma fisica
liquid
Confezionamento
pkg of 1 × 5 mL (800819C-10mg)
Produttore/marchio commerciale
Avanti Research™ - A Croda Brand 800819C
Concentrazione
2 mg/mL (800819C-10mg)
Tipo di lipide
neutral lipids
neutral glycerides
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
Categorie correlate
Descrizione generale
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Applicazioni
- in the preparation of Golgi-like liposomes
- to study its effect on conventional protein kinase C (cPKC) and novel protein kinase C (nPKC) isozymes in vitro
- as a substrate for the measurement of diacylglycerol kinase η1 (DGKη1) activity in vitro
Confezionamento
Stoccaggio e stabilità
Altre note
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Organi bersaglio
Central nervous system, Liver,Kidney
Classe di pericolosità dell'acqua (WGK)
WGK 3
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.