W431300
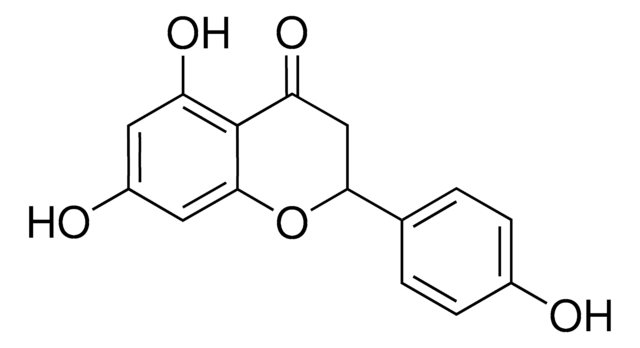
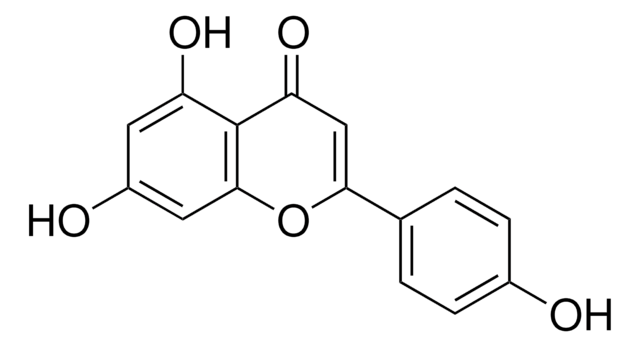
Hesperetin
≥95%
Sinonimo/i:
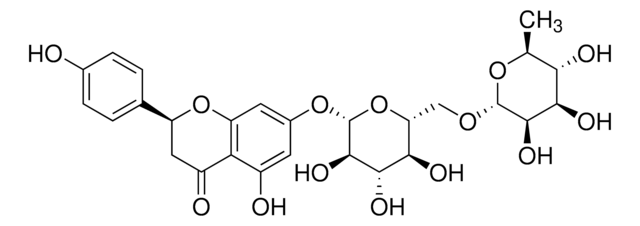
3′,5,7-Trihydroxy-4′-methoxyflavanone, 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-chromanone
About This Item
Prodotti consigliati
Origine biologica
synthetic
Saggio
≥95%
Forma fisica
powder (with a light tan cast)
Punto di fusione
227-232 °C
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Organolettico
odorless
Stringa SMILE
COc1ccc(cc1O)C2CC(=O)c3c(O)cc(O)cc3O2
InChI
1S/C16H14O6/c1-21-13-3-2-8(4-10(13)18)14-7-12(20)16-11(19)5-9(17)6-15(16)22-14/h2-6,14,17-19H,7H2,1H3
AIONOLUJZLIMTK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Esclusione di responsabilità
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.