W200808
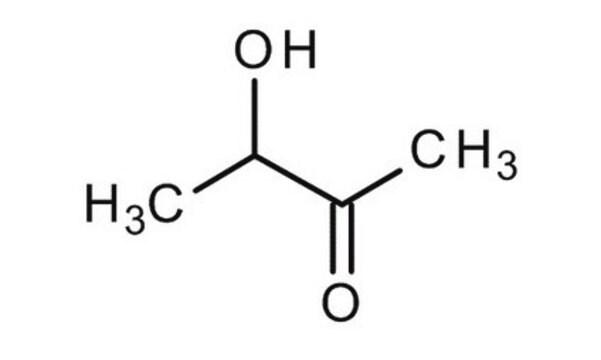
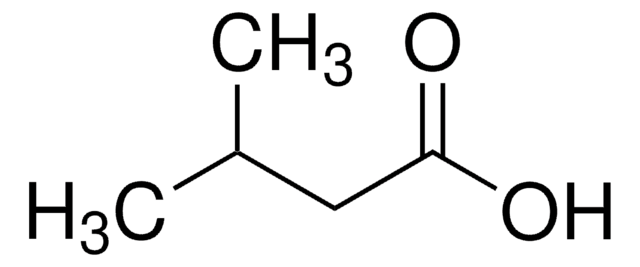
Acetoin
primarily dimer, ≥95%, FG
Sinonimo/i:
3-Hydroxy-2-butanone, Acetylmethylcarbinol
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Grado
FG
Fragrance grade
Halal
Kosher
agenzia
follows IFRA guidelines
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 182.60
Saggio
≥95%
Indice di rifrazione
n20/D 1.417 (lit.)
P. ebollizione
148 °C (lit.)
Punto di fusione
15 °C (monomer)
90 °C (dimer) (lit.)
Solubilità
acetone: soluble(lit.)
water: soluble(lit.)
Densità
1.013 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Organolettico
butter; creamy; cheesy
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(O)C(C)=O
InChI
1S/C4H8O2/c1-3(5)4(2)6/h3,5H,1-2H3
ROWKJAVDOGWPAT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Role of Volatile Organic Compounds Produced by Kosakonia cowanii Cp1 during Competitive Colonization Interaction against Pectobacterium aroidearum SM2.: This study investigates the role of volatile organic compounds, including acetoin, produced by Kosakonia cowanii Cp1 in inhibiting Pectobacterium aroidearum SM2, highlighting the potential of acetoin in biocontrol applications (Mena Navarro et al., 2024).
- Chemical imitation of yeast fermentation by the drosophilid-pollinated deceptive trap-flower Aristolochia baetica (Aristolochiaceae).: The research explores how Aristolochia baetica mimics yeast fermentation, including the production of acetoin, to attract drosophilid pollinators, emphasizing acetoin′s role in plant-pollinator interactions (Rupp et al., 2024).
- Investigating the impact of various sorghum types on the key aroma compounds of Sichuan Xiaoqu Baijiu through application of the sensomics approach.: This study examines how different sorghum types influence the key aroma compounds, including acetoin, in Sichuan Xiaoqu Baijiu, demonstrating acetoin′s significance in food and beverage flavor profiles (Ma et al., 2024).
- Regulation of Tetramethylpyrazine Formation by the Phenolics-Fenton Coupled Redox Cycling System.: The research delves into the biochemical pathways regulated by acetoin in the formation of tetramethylpyrazine, providing insights into its role in flavor compound biosynthesis (Xu et al., 2024).
- Design of a synthetic enzyme cascade for the in vitro fixation of formaldehyde to acetoin.: This paper presents the development of a synthetic enzyme cascade to convert formaldehyde to acetoin, showcasing its potential in biotechnological applications for formaldehyde detoxification (Cui et al., 2024).
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
4.1B - Flammable solid hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W200808-1KG | |
| W200808-25KG | |
| W200808-25KG-K | 4061837806476 |
| W200808-SAMPLE | |
| W200808-1KG-K | 4061837806469 |
| W200808-5KG | |
| W200808-5KG-K | 4061837806483 |
| W200808-SAMPLE-K | 4061837488948 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.