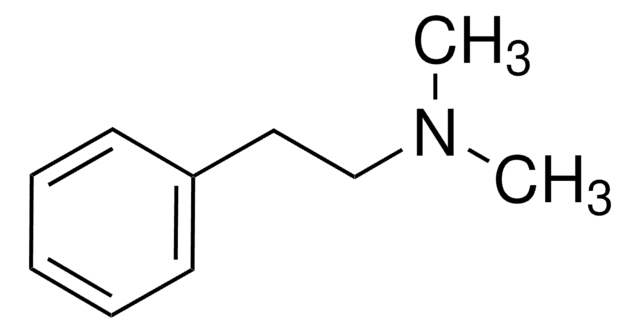
M68423
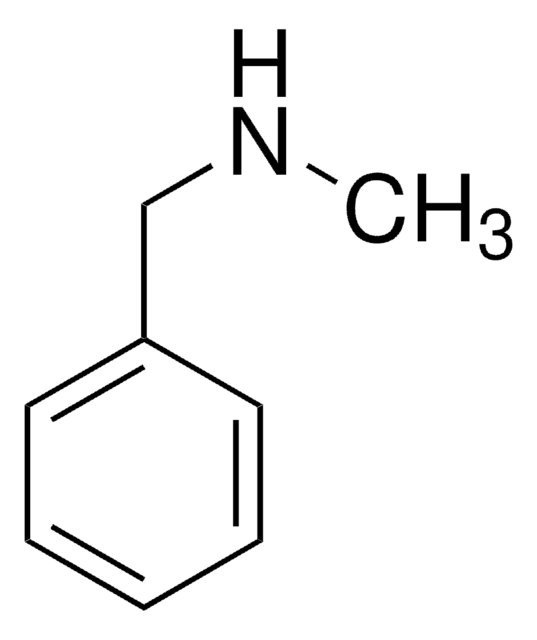
N-Methyl-phenethylamine
99%
Sinonimo/i:
N-Methyl-2-phenylethylamine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5CH2CH2NHCH3
Numero CAS:
Peso molecolare:
135.21
Beilstein:
636347
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.516 (lit.)
P. eboll.
203 °C (lit.)
Densità
0.93 g/mL at 25 °C (lit.)
Stringa SMILE
CNCCc1ccccc1
InChI
1S/C9H13N/c1-10-8-7-9-5-3-2-4-6-9/h2-6,10H,7-8H2,1H3
SASNBVQSOZSTPD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
N-Methyl-phenethylamine can be used as a reactant:
- To synthesize N-methyl-phenethylamine based tertiary amines by reacting with different alkyl halides in the presence of triphenylphosphine (TPP) and diisopropylazocarboxylate (DIAD) via N-alkylation reaction.
- To fabricate photochemically stable, super-sensitive, and highly selective fluorescent film for the detection of N-methamphetamine (an illicit drug).
- To prepare biologically active squaric acid N-hydroxylamide amide derivatives.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Efficient synthesis of tertiary amines from secondary amines
Michio K, et al.
Tetrahedron Letters, 47, 4871-4875 (2006)
Fabrication of a new fluorescent film and its superior sensing performance to N-methamphetamine in vapor phase
H Meixia, et al.
Sensors and Actuators B, Chemical, 227 (2016)
T D Forbes et al.
Journal of animal science, 72(2), 464-469 (1994-02-01)
Eighteen Suffolk and Suffolk x Hampshire wethers (56.3 +/- 1.3 kg) were used to determine the effects of naturally occurring amines, N-methyl-beta-phenethylamine (NMP) and tyramine (T), on plasma cortisol, norepinephrine (NE), ACTH, and GnRH-stimulated LH concentrations. In each experiment, wethers
J D Duncan et al.
Drug metabolism and disposition: the biological fate of chemicals, 11(1), 15-20 (1983-01-01)
The effects of methyl, ethyl, isopropyl, isobutyl, and benzyl substituents at the alpha-carbon of N-methyl-2-phenethylamine on the kinetics of its N-demethylation in liver microsomes from both control and phenobarbital pretreated rats were studied. In control microsomes, the kinetic studies indicated
I Osamu
European journal of nuclear medicine, 8(9), 385-388 (1983-01-01)
A new type of metabolically trapped agent for measuring regional brain function was designed and evaluated. N-methylphenylethylamine (14C-MPEA) was synthesized with trifluoroacetylphenylethylamine and 14C-methyl iodide. A high concentration of 14C-MPEA accumulated in mouse brain 1 min after injection, and radioactivities
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.