H36605
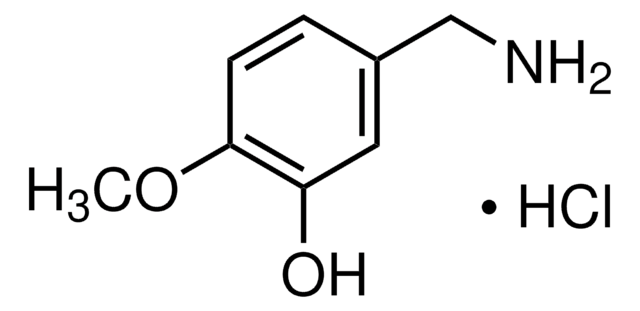
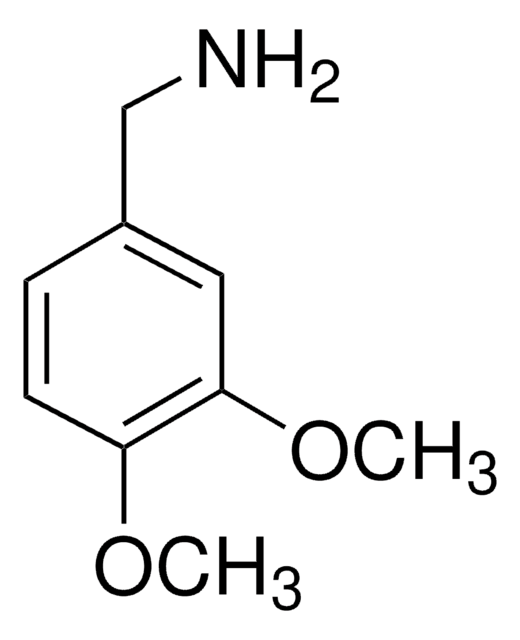
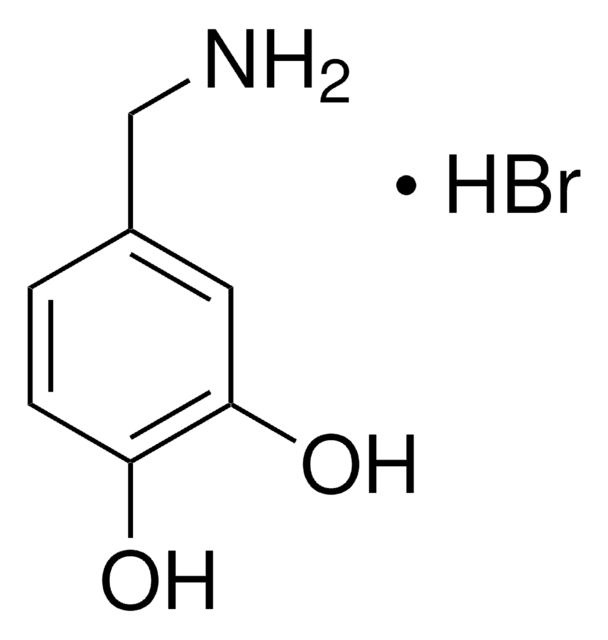
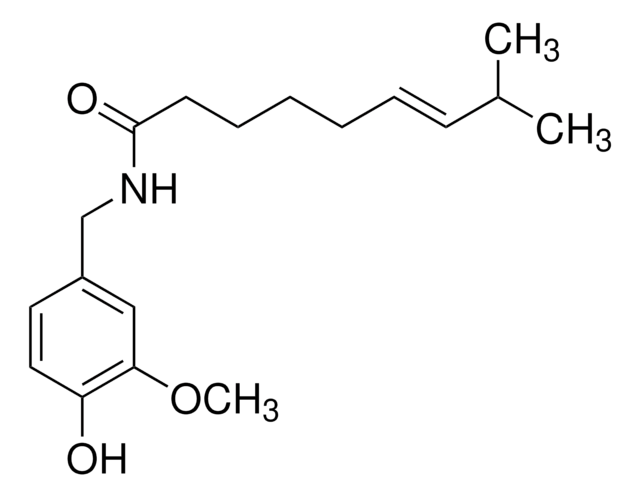
4-Hydroxy-3-methoxybenzylamine hydrochloride
98%
Sinonimo/i:
Vanillylamine hydrochloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HOC6H3(OCH3)CH2NH2 · HCl
Numero CAS:
Peso molecolare:
189.64
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
219-221 °C (dec.) (lit.)
Stringa SMILE
Cl.COc1cc(CN)ccc1O
InChI
1S/C8H11NO2.ClH/c1-11-8-4-6(5-9)2-3-7(8)10;/h2-4,10H,5,9H2,1H3;1H
PUDMGOSXPCMUJZ-UHFFFAOYSA-N
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Harishchandra B Gururaj et al.
Plant science : an international journal of experimental plant biology, 195, 96-105 (2012-08-28)
Capsaicinoid biosynthesis involves the participation of two substrates viz. vanillylamine and C(9)-C(11) fatty acid moieties. Vanillylamine which is a derivative of vanillin is synthesized through a transaminase reaction in the phenylpropanoid pathway of capsaicinoid synthesis. Here we report the functional
Bellur Chayapathy Narasimha Prasad et al.
Journal of agricultural and food chemistry, 54(18), 6660-6666 (2006-08-31)
Capsaicin, a pungency factor alkaloid of Capsicum fruits, is biosynthesized by enzymatic condensation of vanillylamine, a phenyl propanoid intermediate, and 8-methyl-nonenoic acid, a fatty acid derivative from the leucine/valine pathway by capsaicin synthase. Biotic elicitors, such as aqueous mycelial extracts
Suvi F Flagan et al.
Environmental microbiology, 8(3), 560-565 (2006-02-16)
Capsaicin contributes to the organoleptic attributes of hot peppers. Here, we show that capsaicin is utilized as a growth nutrient by certain bacteria. Enrichment cultures utilizing capsaicin were successfully initiated using Capsicum-derived plant material or leaves of tomato (a related
Yaqin Lang et al.
The Plant journal : for cell and molecular biology, 59(6), 953-961 (2009-05-29)
Capsaicinoids are responsible for the spicy flavor of pungent peppers (Capsicum). The cultivar CH-19 Sweet is a non-pungent pepper mutant derived from a pungent pepper strain, Capsicum annuum CH-19. CH-19 Sweet biosynthesizes capsaicinoid analogs, capsinoids. We determined the genetic and
Kenji Kobata et al.
Bioscience, biotechnology, and biochemistry, 75(8), 1611-1614 (2011-08-09)
Stable isotope-labeled precursors were synthesized for an analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS) to elucidate the biosynthetic flow of capsaicinoids, capsinoids, and capsiconinoids. [1'-(13)C][5-(2)H]-Vanillin was prepared by the condensation of guaiacol with [(13)C]-chloroform and a D(2)O treatment. Labeled vanillylamine
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.