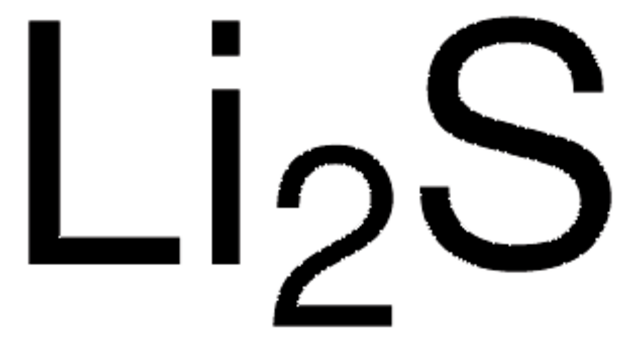930946
Lithium nitrate
battery grade, ≥99.9% trace metals basis
Sinonimo/i:
Lithium salt of nitric acid
About This Item
Prodotti consigliati
Livello qualitativo
Grado
battery grade
Saggio
≥99.9% trace metals basis
Stato
powder
Caratteristiche più verdi
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
≤0.5 wt. % H2O
≤1000 ppm (trace metals analysis)
Punto di fusione
264 °C (lit.)
Solubilità
H2O: soluble (highly soluble(lit.))
acetone: soluble ((lit.))
alcohols: soluble ((lit.))
Anioni in tracce
chloride (Cl-): ≤500 ppm
sulfate (SO42-): ≤200 ppm
applicazioni
battery manufacturing
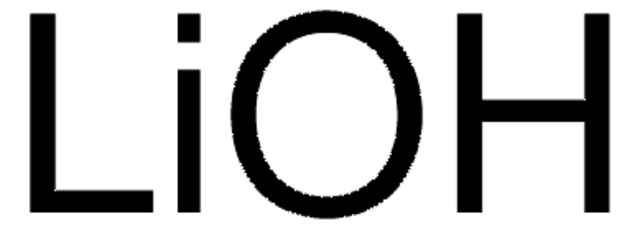
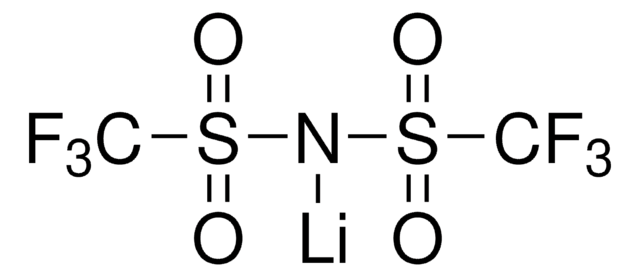
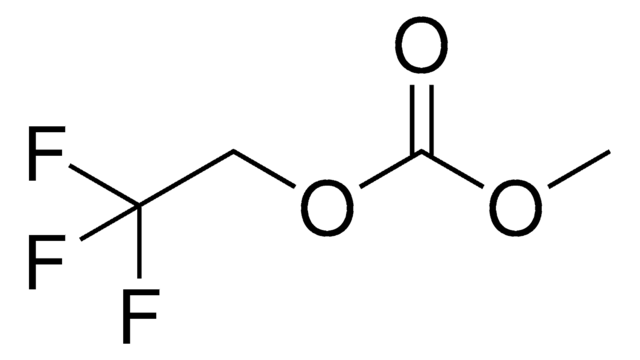
Categoria alternativa più verde
Stringa SMILE
[Li+].[O-][N+]([O-])=O
InChI
1S/Li.NO3/c;2-1(3)4/q+1;-1
IIPYXGDZVMZOAP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Lithium nitrate is produced by the acid-base reaction between nitric acid and lithium carbonate, which evolves carbon dioxide and water. The resulting material is dried, purified, and heated to form the anhydrous product.
Applicazioni
Because lithium nitrate is soluble in water, researchers also use lithium nitrate in the synthesis of lithium compounds using a host of solution-based chemistries. For example, microwave-induced combustion using solutions of lithium nitrate has yielded olivine-type lithium iron phosphate (LiFePO4), lithium cobalt oxide (LiCoO2), and lithium titanium oxides (ex. Li4Ti5O12 and Li2TiO3). Hydrothermal processing, sol-gel processing, spray pyrolysis, co-precipitation pre-processing, and Li emulsion-drying methods have all used lithium nitrate as a reactant to form lithium metal oxides. These techniques can yield controlled particle size, grain size, crystallinity, or facilitate the introduction of dopants for engineering the properties of the products, often explored for next-generation lithium-ion batteries.
Our battery grade lithium nitrate with ≥99.9% trace metals purity and low chloride and sulfate impurities, is designed as a precursor for cathode materials for lithium-ion batteries.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 3
Codice della classe di stoccaggio
5.1B - Oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.