90827
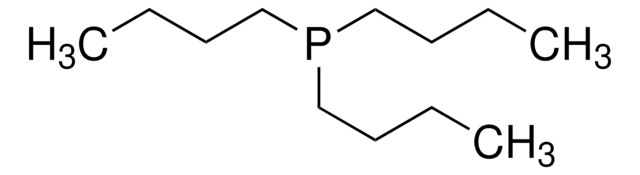
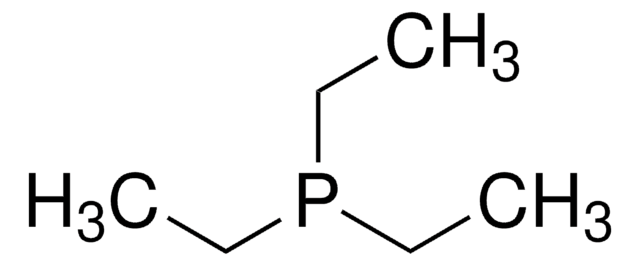
Tributylphosphine
≥93.5% (Tri-N-butylphosphine, GC)
Sinonimo/i:
P(n-Bu)3, TBP
About This Item
Prodotti consigliati
Densità del vapore
9 (vs air)
Livello qualitativo
Saggio
≥93.5% (Tri-N-butylphosphine, GC)
≥97% (Tri-N-butylphospine + isomers)
Forma fisica
liquid
Temp. autoaccensione
392 °F
Impiego in reazioni chimiche
reaction type: Acetylations
reagent type: ligand
Indice di rifrazione
n20/D 1.462 (lit.)
n20/D 1.463
P. eboll.
150 °C/50 mmHg (lit.)
Densità
0.81 g/mL at 25 °C (lit.)
Gruppo funzionale
phosphine
Stringa SMILE
CCCCP(CCCC)CCCC
InChI
1S/C12H27P/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
TUQOTMZNTHZOKS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
It may be used in the following processes:
- As reducing agent for alkyl disulfides and aromatic disulfides.
- As catalyst for the synthesis of 2-substituted 1,3-benzoselenazoles.
- As promoter for the ring opening of epoxides and aziridines with nucleophiles.
- As a reagent in the preparation of 6-substituted penicillanate esters by reduction of 6-bromo-6-substituted penicillanate esters in high diastereoselectivity.
- As a catalyst in the acylation reaction of alcohols.
- As a catalyst to prepare rotaxanes by the acylation of corresponding pseudorotaxanes using 3,5-dimethylbenzoic anhydride.
- As a catalyst to prepare vinyl thioethers by the Michael addition of ethanethiol to various alkynyl ketones.
- As a promoter in the conjugate addition of non-nucleophilic N-containing compounds with Michael acceptors.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1A
Codice della classe di stoccaggio
4.2 - Pyrophoric and self-heating hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
242.6 °F - closed cup
Punto d’infiammabilità (°C)
117 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.