803383
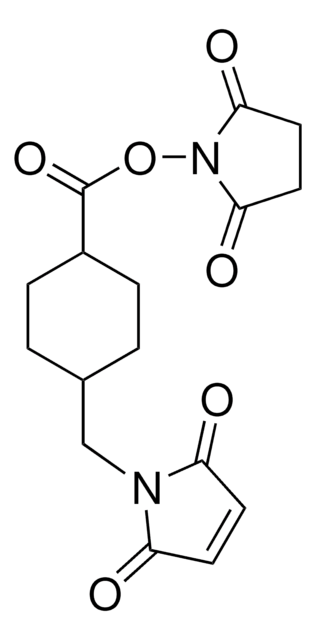
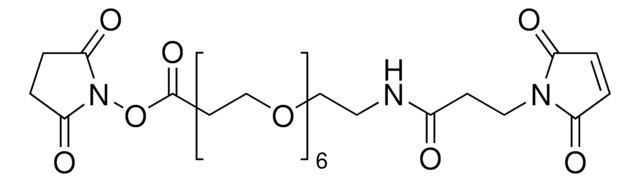
LC-SMCC (succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxy-(6-amidocaproate))
About This Item
Prodotti consigliati
Saggio
≥90%
Livello qualitativo
Forma fisica
powder
PM
447.48
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Condizioni di stoccaggio
desiccated
Solubilità
DMSO or DMF: soluble
Gruppo funzionale
NHS ester
maleimide
Condizioni di spedizione
ambient
Temperatura di conservazione
2-8°C
Stringa SMILE
O=C(CCC1=O)N1OC(CCCCCNC(C2CCC(CN3C(C=CC3=O)=O)CC2)=O)=O
InChI
1S/C22H29N3O7/c26-17-9-10-18(27)24(17)14-15-5-7-16(8-6-15)22(31)23-13-3-1-2-4-21(30)32-25-19(28)11-12-20(25)29/h9-10,15-16H,1-8,11-14H2,(H,23,31)
IHVODYOQUSEYJJ-UHFFFAOYSA-N
Descrizione generale
Caratteristiche e vantaggi
- Reactive groups: NHS ester and maleimide
- Reactive toward: amino and sulfhydryl groups
- Long-chain variety of SMCC
- High purity, crystalline reagent can be used to create high-purity maleimide-activated derivatives
- Non-cleavable
- Water-insoluble (dissolve first in DMF or DMSO)
- Cyclohexane bridge confers added stability to the maleimide group making LC-SMCC an ideal crosslinking agent for maleimide activation of proteins. Maleimide groups are stable for 64 hours in 0.1 M sodium phosphate buffer, pH 7 at 4°C.
Avvertenza
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

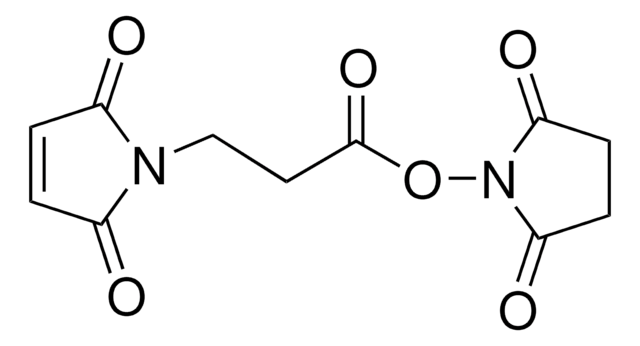


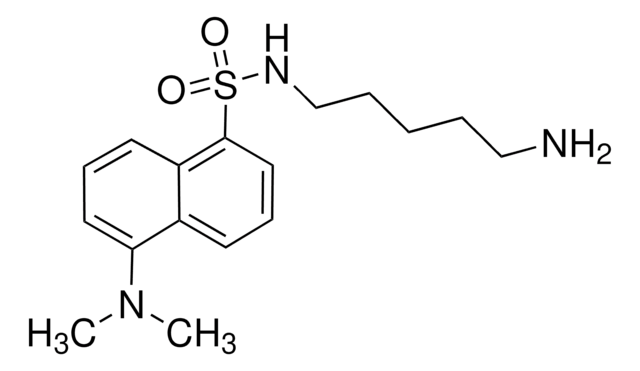
![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)



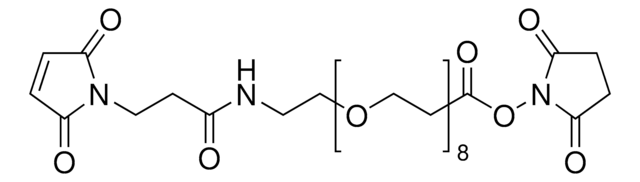
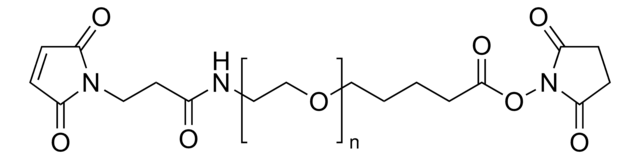
![O-[N-(6-Maleimidohexanoyl)aminoethyl]-O′-[3-(N-succinimidyloxy)-3-oxopropyl]polyethylene glycol 3,000 Mp 3,000](/deepweb/assets/sigmaaldrich/product/structures/296/558/8fba6773-c240-41a2-ac1d-15c2185ceddd/640/8fba6773-c240-41a2-ac1d-15c2185ceddd.png)