79330
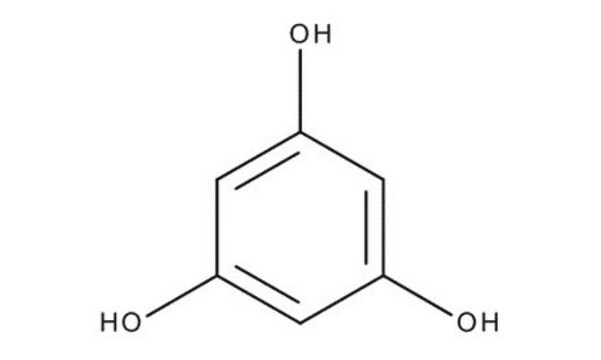
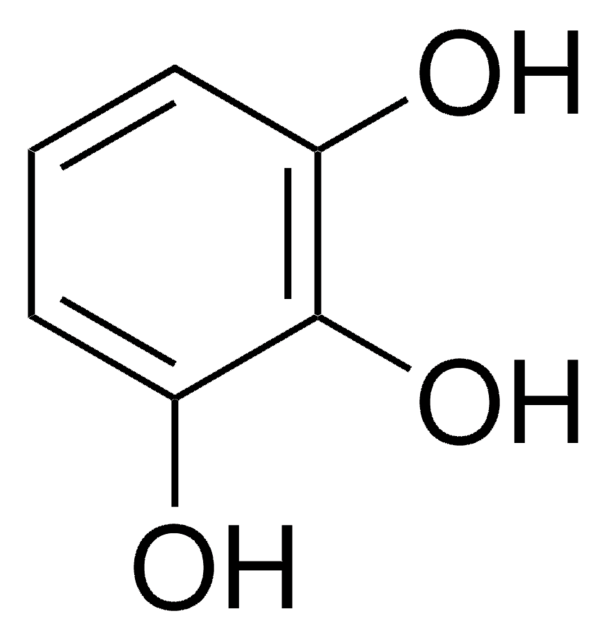
Phloroglucinol
≥99.0% (HPLC)
Sinonimo/i:
1,3,5-Trihydroxybenzene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H6O3
Numero CAS:
Peso molecolare:
126.11
Beilstein:
1341907
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥99.0% (HPLC)
Stato
solid
Impurezze
diresorcin, none detected
≤2% water
Punto di fusione
215-220 °C
Stringa SMILE
Oc1cc(O)cc(O)c1
InChI
1S/C6H6O3/c7-4-1-5(8)3-6(9)2-4/h1-3,7-9H
QCDYQQDYXPDABM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Phloroglucinol (phlo) is a phenol derivative that shows cyctoprotective effect from oxidative damage by enhancing the activity of cellular catalase.
- It can react with benzaldehyde derivatives to form phloroglucinol-based microporous polymeric organic frameworks (phlo-POF) with potential applications in ion-exchange and gas adsorption.
- Phlo can also be used to prepare synthetic analogs of A-type proanthocyanidins (PACs) such as 2,8-dioxabicyclo[3.3.1]nonane derivatives by reacting with the corresponding flavylium salts.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation
Kang KA, et al.
Journal of Cellular Biochemistry, 97(3), 609-620 (2006)
Efficient and Rapid Photocatalytic Reduction of Hexavalent Chromium Achieved by a Phloroglucinol-Derived Microporous Polymeric Organic Framework Solid
Kostas V, et al.
The Journal of Physical Chemistry C, 121(13), 7303-7311 (2017)
Thermodynamic Stability of Flavylium Salts as a Valuable Tool To Design the Synthesis of A-Type Proanthocyanidin Analogues
Alejo-Armijo A, et al.
The Journal of Organic Chemistry, 83(19), 12297-12304 (2018)
Claudia Birkemeyer et al.
Metabolites, 10(9) (2020-09-17)
Accumulation of biologically active metabolites is a specific feature of plant biochemistry, directing the use of plants in numerous applications in the pharmaceutical and food industries. Among these substances, the plethora of phenolic compounds has attracted particular interest among researchers.
Anne M Vissers et al.
Phytochemical analysis : PCA, 28(6), 487-495 (2017-06-15)
Phlorotannins are complex mixtures of phloroglucinol oligomers connected via C-C (fucols) or C-O-C (phlorethols) linkages. Their uniformity in subunits and large molecular weight hamper their structural analysis. Despite its commercial relevance for alginate extraction, phlorotannins in Laminaria digitata have not
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.