673854
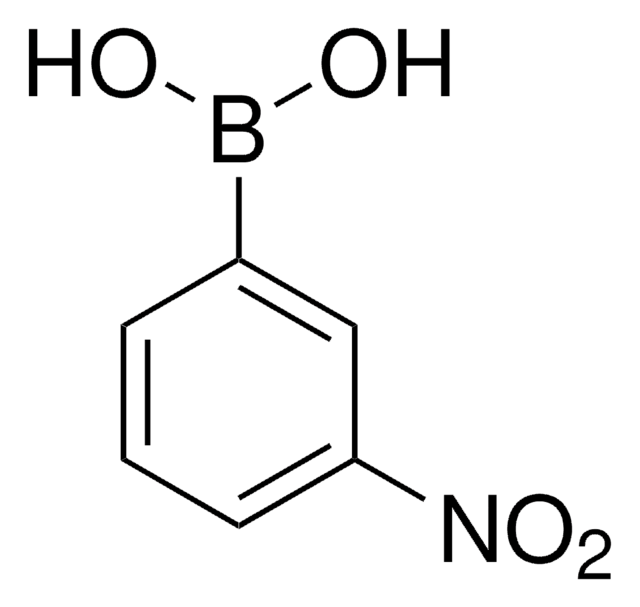
4-Nitrophenylboronic acid
≥95.0%
Sinonimo/i:
4-Nitrobenzeneboronic acid, p-Nitrophenylboronic acid, p-nitro-benzeneboronic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(O2N)C6H4(B(OH)2)
Numero CAS:
Peso molecolare:
166.93
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥95.0%
Stato
solid
Punto di fusione
285-290 °C (dec.)
Gruppo funzionale
nitro
Stringa SMILE
OB(O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H6BNO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,9-10H
NSFJAFZHYOAMHL-UHFFFAOYSA-N
Applicazioni
Reagent used for
Reagent used in Preparation of
- Ligand-free palladium-catalyzed Suzuki-Miyaura cross-couplings
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines
- Diels-Alder or C-H activation reactions
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulations
- N-arylation of phenylurea using copper acetylacetonate catalyst
- Environmentally benign one-pot synthesis through a double arylation process
- Copper-mediated cyanations
- copper-catalyzed arylations
- Regioselective glycosylations
- Suzuki couplings followed by arylations
- X-ray absorption on rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Reagent used in Preparation of
- Combretastatin analogs as potential antitumor agents
- Human immunodeficiency virus (HIV) protease inhibitors with antiviral activities against drug-resistant viruses
Altre note
May contain varying amounts of anhydride
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Motokura, K.; et al.
Green Chemistry, 13, 2416-2422 (2011)
An efficient copper-catalyzed one-pot synthesis of diaryl thioethers by coupling of arylboronic acids with potassium ethyl xanthogenate under mild conditions
Wang, L.; et al.
Synlett, 20, 3041-3045 (2011)
An efficient access to 2,3-diarylimidazo[1,2-a]pyridines via imidazo[1,2-a]pyridin-2-yl triflate through a Suzuki cross-coupling reaction-direct arylation sequence
Marhadour, S.; et al.
Tetrahedron Letters, 53, 297-300 (2012)
Abdallah Hamze et al.
ChemMedChem, 6(12), 2179-2191 (2011-10-13)
A novel class of isocombretastatin A-4 (isoCA-4) analogues with modifications at the 3'-position of the B-ring by replacement with C-linked substituents was studied. Exploration of the structure-activity relationships of theses analogues led to the identification of several compounds that exhibit
Tetrahedron, 63, 6131-6131 (2007)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.