597988
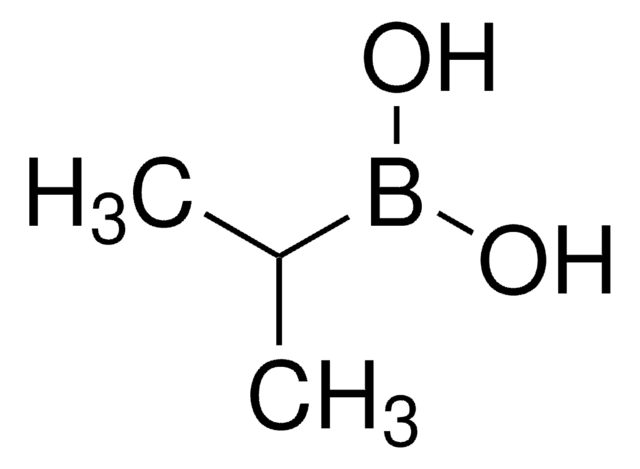
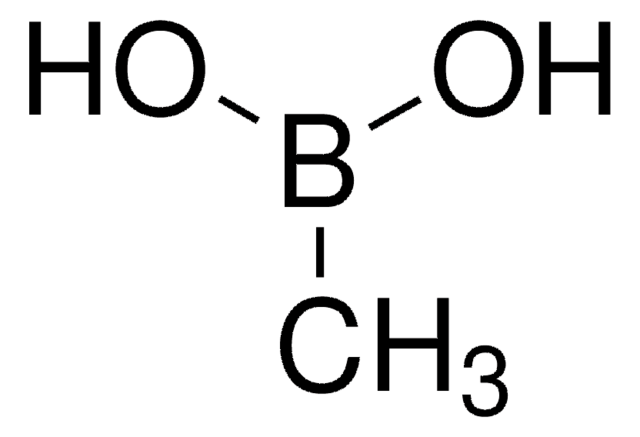
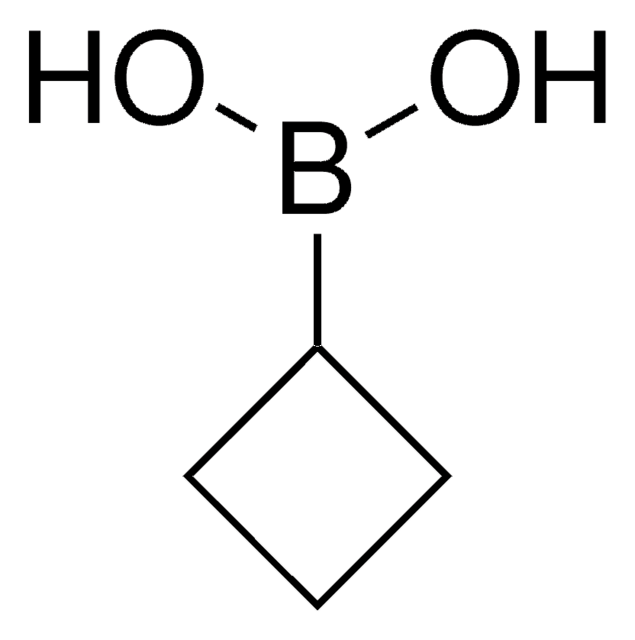
Cyclopropylboronic acid
Sinonimo/i:
Cyclopropaneboronic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C3H7BO2
Numero CAS:
Peso molecolare:
85.90
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Stato
solid
Punto di fusione
90-95 °C (lit.)
Temperatura di conservazione
−20°C
Stringa SMILE
OB(O)C1CC1
InChI
1S/C3H7BO2/c5-4(6)3-1-2-3/h3,5-6H,1-2H2
WLVKDFJTYKELLQ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Boronic acid component in a study of carbon-hydrogen bond alkylation in the presence of Pd(II), Ag(I) and benzoquinone.
Cu-mediated N-cyclopropanation
Reagent used for
Reagent used in Preparation of
- Microwave-assisted copper(II)-catalyzed N-cyclopropylation
- Nickel- and copper-catalyzed Suzuki-Miyaura coupling reaction of arenes
- Palladacycle-catalyzed Suzuki-cross coupling of aryl halides with cyclopropylboronic acid
- Palladium(0)-catalyzed cyclopropane C-H bond functionalization
- Palladium-catalyzed decarboxylative coupling
- Palladium-catalyzed ligand-directed oxidative functionalization of cyclopropanes
- Palladium-catalyzed Suzuki coupling reaction
Reagent used in Preparation of
- Diaryl ketones by arylation of arylboronic acids with aromatic aldehydes catalyzed by Cu(OTf)2 and Xantphos
- Aminothiazolylpyrrolidine-based tartrate diamides as TACE inhibitors to treat inflammatory disorders and cancer
Altre note
Contains varying amounts of anhydride
May contain 3-5% cyclopropanol
May contain 5-10% boric acid
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
A Mild Palladium-Catalyzed Suzuki Coupling Reaction of Quinoline Carboxylates with Boronic Acids
W. Li, et al.,
Advanced Synthesis & Catalysis, 353, 1671-1675 (2011)
Ni- and Cu-catalyzed coupling reactions using 2-(4,5-dihydro-1H-imidazo-2-yl)phenol as a versatile phosphine-free ligand
Haneda, S.; Sudo, K.; Hayashi, M.
Heterocycles, 84, 569-575 (2012)
Xiao Chen et al.
Journal of the American Chemical Society, 128(39), 12634-12635 (2006-09-28)
Palladium-catalyzed alkylations of sp2 and sp3 C-H bonds with either methylboroxine or alkylboronic acids were developed. Ag2O or AgCO3 is used as a crucial oxidant and promoter for the transmetalation step. Ether, ester, alcohol, and alkene functional groups are tolerated.
Copper-catalyzed arylation of arylboronic acids with aldehydes
Zheng, H.; et al.
Synlett, 11, 1626-1630 (2011)
Microwave-assisted N-cyclopropylation of pyridinols with cyclopropyl boronic acid
Tambe, Y. B.; et al.
Synthetic Communications, 42, 1341-1348 (2012)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)