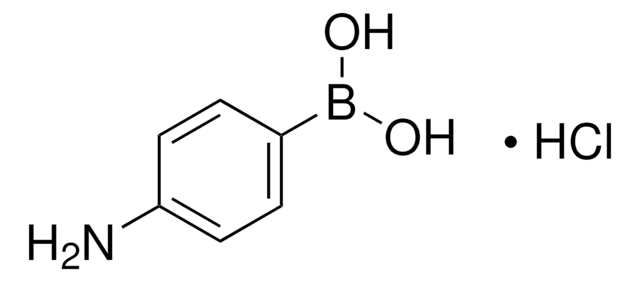
523976
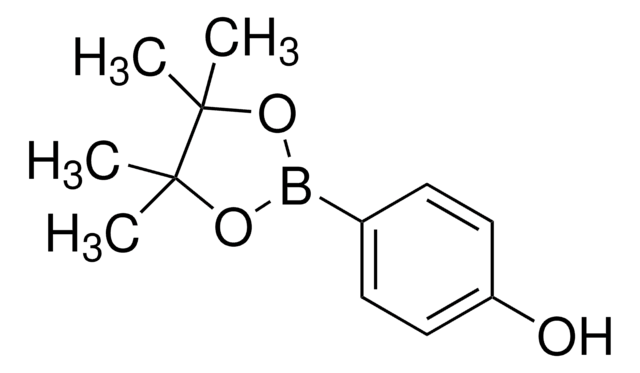
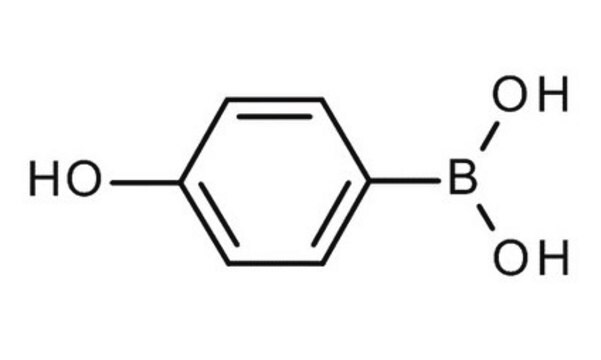
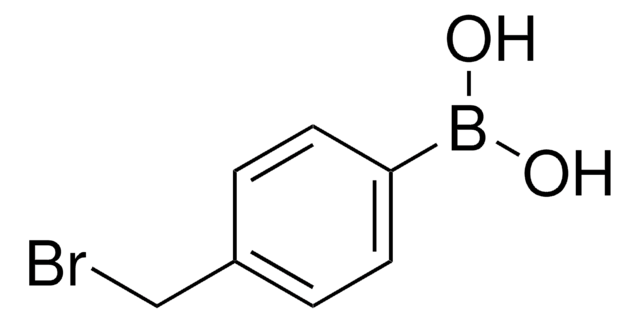
4-Hydroxyphenylboronic acid
≥95.0%
Sinonimo/i:
(p-Hydroxyphenyl)boronic acid, 4-Hydroxybenzeneboronic acid, p-hydroxy-benzeneboronic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HOC6H4B(OH)2
Numero CAS:
Peso molecolare:
137.93
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0%
Stato
solid
Punto di fusione
>230 °C (lit.)
Stringa SMILE
OB(O)c1ccc(O)cc1
InChI
1S/C6H7BO3/c8-6-3-1-5(2-4-6)7(9)10/h1-4,8-10H
COIQUVGFTILYGA-UHFFFAOYSA-N
Categorie correlate
Applicazioni
4-Hydroxyphenylboronic acid can be used as a reactant in:
It can also be used to prepare/promote:
- Suzuki-Miyaura coupling and Stille coupling reactions.
- Palladium-catalyzed aminocarbonylation and cross-coupling reactions.
- Suzuki reaction for preparation of bio-supported palladium nanoparticles as phosphine-free catalysts.
- Cu2O-catalyzed aerobic oxidative cross-coupling of tetrazoles.
It can also be used to prepare/promote:
- PDK1 inhibitory activity (cancer cell growth, survival, and tumorigenesis inhibitor).
- Rod-like dendronized polymers containing G4 and G5 ester dendrons via macromonomer approach by living ROMP.
- Estrone-derived cyclopamine analogs as Sonic Hedgehog signaling inhibitors for anti-cancer chemotherapeutics.
- Enzymatic inhibitors for the treatment of Gram-negative bacterial infections.
- Oligoarenes by Suzuki-Miyaura palladium-catalyzed cross-coupling.
Altre note
Contains varying amounts of anhydride
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Zhaoyang Lu et al.
Soft matter, 12(17), 3860-3867 (2016-03-31)
The self-assembling behavior of coil-rod-coil molecules 1a, 1b, and 2a, 2b was investigated using DSC, POM, SAXS, and AFM in bulk and aqueous solutions. These molecules contain p-quinquephenyl groups as rod segments incorporating lateral hydroxyl or methoxyl groups in the
Stéphanie Blanchard et al.
Bioorganic & medicinal chemistry letters, 22(8), 2880-2884 (2012-03-23)
A series of 2-anilino substituted 4-aryl-8H-purines were prepared as potent inhibitors of PDK1, a serine-threonine kinase thought to play a role in the PI3K/Akt signaling pathway, a key mediator of cancer cell growth, survival and tumorigenesis. The synthesis, SAR and
Synthetic approach to the chemical isostere of O-methyl honokiol
Cui, M.; Kim, H. S.
Synlett, 23, 311-313 (2012)
Highly selective palladium-catalyzed aminocarbonylation and cross-coupling reactions on a cavitand scaffold
Csok, Z.; Takatsy, A.; Kollar, L.
Tetrahedron, 68, 2657-2661 (2012)
Synthesis of Rod-Like Dendronized Polymers Containing G4 and G5 Ester Dendrons via Macromonomer Approach by Living ROMP
Kim, K.O.; Choi, T-L.
ACS Macro Letters, 1, 445-448 (2012)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 523976-5G | 4061832549460 |
| 523976-1G | 4061832549354 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.