512125
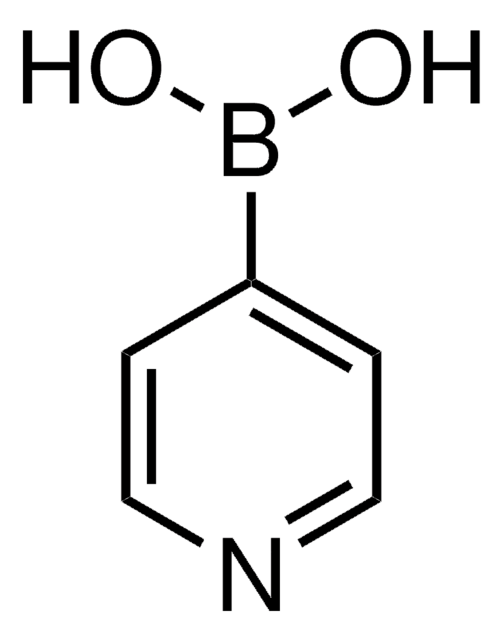
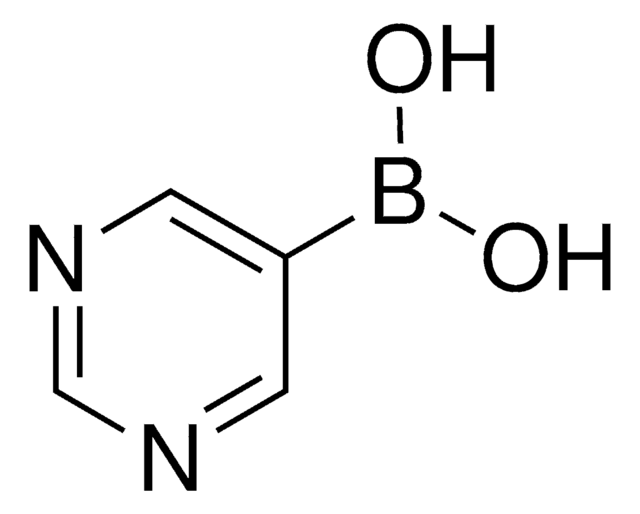
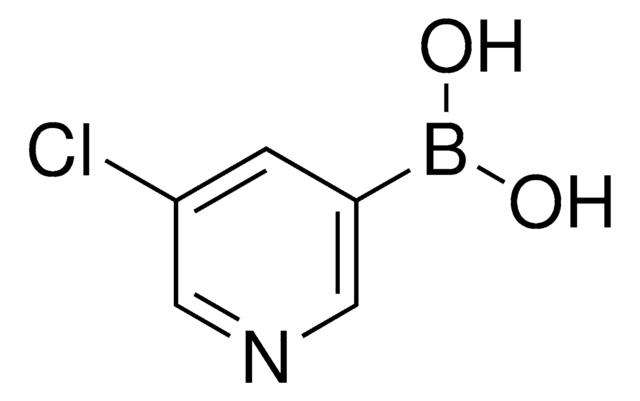
3-Pyridinylboronic acid
≥95.0%
Sinonimo/i:
3-Pyridineboronic acid, 3-Pyridylboronic acid, Dihydroxy(3-pyridyl)borane, Pyridin-3-ylboronic acid
About This Item
Prodotti consigliati
Saggio
≥95.0%
Stato
solid
Punto di fusione
>300 °C (lit.)
Stringa SMILE
OB(O)c1cccnc1
InChI
1S/C5H6BNO2/c8-6(9)5-2-1-3-7-4-5/h1-4,8-9H
ABMYEXAYWZJVOV-UHFFFAOYSA-N
Categorie correlate
Applicazioni
- Phosphine-free Suzuki-Miyaura cross-coupling reactions.
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation.
- N-arylation using copper acetylacetonate catalyst.
- Copper-mediated cyanation and regioselective cyanation of electron-rich benzenes.
It can also be used to prepare:
- New linear poly(phenylpyridyl) chains by Suzuki coupling.
- Oligopyridyl foldamers as mimics of a-helix twist.
- Many highly significant therapeutic enzymatic and kinase inhibitors and receptor antagonists.
- Pyridine substituted pyridinium N-(2′-azinyl)aminides by reacting with dibromo pyridinium aminides via Suzuki coupling reaction.
Altre note
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.