49800
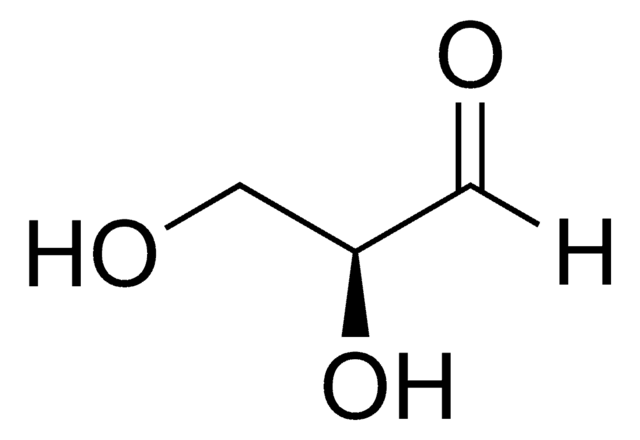
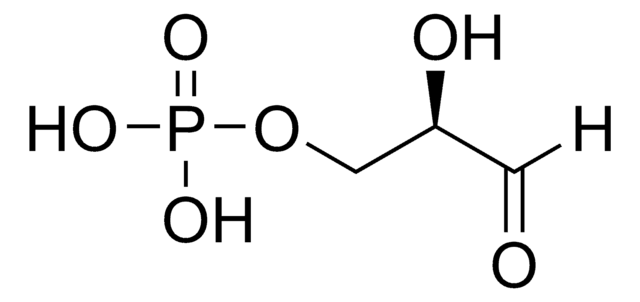
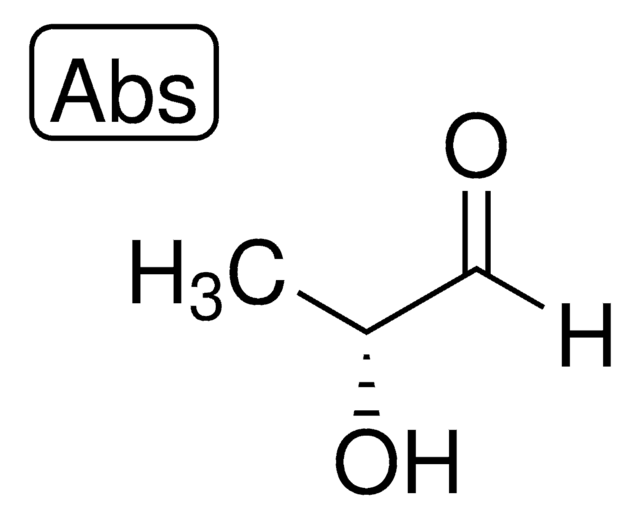
D-(+)-Glyceraldehyde
≥98.0% (HPLC)
Sinonimo/i:
(2R)-2,3-Dihydroxypropanal, Triose
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C3H6O3
Numero CAS:
Peso molecolare:
90.08
Beilstein:
1720474
Numero CE:
Numero MDL:
Codice UNSPSC:
12352200
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥98.0% (HPLC)
Impurezze
≤10% water
Temperatura di conservazione
2-8°C
Stringa SMILE
OC[C@@H](O)C=O
InChI
1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2/t3-/m0/s1
MNQZXJOMYWMBOU-VKHMYHEASA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
D-(+)-Glyceraldehyde can be utilized as a reactant in the synthesis of:
- (S)-homophenylalanine by ruthenium oxidation of a 3-amino-1,2-diol generated via coupling of an amine, and α-hydroxyaldehyde.
- β- and γ-allenols via metal-catalyzed cyclization. Allenols are used as a key precursor for the preparation of enantiopure dihydropyrans and tetrahydrooxepines.
- Isopropylidene D-glyceraldehyde intermediate, which controls the chirality in the total synthesis of prostaglandins (PGE1).
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes
Petasis NA and Zavialov IA
Journal of the American Chemical Society, 120(45), 11798-11799 (1998)
Chiral synthesis of prostaglandins (PGE1) from D-glyceraldehyde
Stork G and Takahashi T
Journal of the American Chemical Society, 99(4), 1275-1276 (1977)
Takayoshi Wakagi et al.
PloS one, 11(1), e0147333-e0147333 (2016-01-26)
Archaea use glycolytic pathways distinct from those found in bacteria and eukaryotes, where unique enzymes catalyze each reaction step. In this study, we isolated three isozymes of glyceraldehyde oxidoreductase (GAOR1, GAOR2 and GAOR3) from the thermoacidophilic archaeon Sulfolobus tokodaii. GAOR1-3
Katarzyna Lechowicz et al.
International journal of molecular sciences, 21(16) (2020-08-13)
Lolium multiflorum/Festuca arundinacea introgression forms have been proved several times to be good models to identify key components of grass metabolism involved in the mechanisms of tolerance to water deficit. Here, for the first time, a relationship between photosynthetic and
Metal-Catalyzed Cyclization of β-and γ-Allenols Derived from d-Glyceraldehyde- Synthesis of Enantiopure Dihydropyrans and Tetrahydrooxepines: An Experimental and Theoretical Study
Alcaide BL
Chemistry?A European Journal, 15(36), 9127-9138 (2009)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.