473863
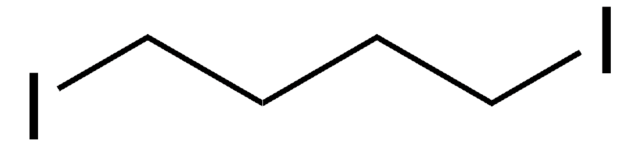
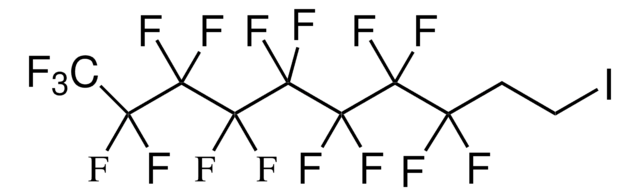
Octafluoro-1,4-diiodobutane
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
I(CF2)4I
Numero CAS:
Peso molecolare:
453.84
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Indice di rifrazione
n20/D 1.429 (lit.)
P. ebollizione
150 °C (lit.)
Punto di fusione
−9 °C (lit.)
Densità
2.474 g/mL at 25 °C (lit.)
Gruppo funzionale
fluoro
iodo
Stringa SMILE
FC(F)(I)C(F)(F)C(F)(F)C(F)(F)I
InChI
1S/C4F8I2/c5-1(6,3(9,10)13)2(7,8)4(11,12)14
JILAKKYYZPDQBE-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Octafluoro-1,4-diiodobutane (ofib, C4F8I2) is also referred to as 1,1,2,2,3,3,4,4-octafluoro-1,4-diiodobutane. It acts as a halogen bond donor compound and its cocrystallization with methyldiphenylphosphine oxide (mdppo) has been investigated. In combination with a calixcrown compound ofib forms a ′binary host′ system, which is employed for the isolation of cesium iodide from aqueous to fluorous phase.
Applicazioni
Octafluoro-1,4-diiodobutane may be used as a guest molecule to prepare a chiral bidentate inclusion complex with pyridyl moieties of a pyridoallenoacetylenic host. It may be employed in the synthesis of three-component supramolecular complexes.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Silvia Castro-Fernández et al.
Organic letters, 16(4), 1136-1139 (2014-02-12)
A chiral bidentate inclusion complex has been formed by halogen-bond interaction between the pyridyl moieties of a pyridoallenoacetylenic host and octafluorodiiodobutane. X-ray crystallography showed that the guest adopts a chiral conformation inside the molecular channels formed by stacking of the
Switching between halogen-and hydrogen-bonding in stoichiometric variations of a cocrystal of a phosphine oxide.
Oh SY, et al.
CrystEngComm, 14(19), 6110-6114 (2012)
Dipyridinocalixcrown/diiodoperfluorocarbon binary host systems for CsI: structural studies and fluorous phase extraction of caesium.
Gattuso G, et al.
Tetrahedron, 63(23), 4951-4958 (2007)
Metric engineering of supramolecular Borromean rings.
Liantonio R, et al.
Chemical Communications (Cambridge, England), 17, 1819-1821 (2006)
Liang Lu et al.
Journal of applied toxicology : JAT, 39(7), 945-954 (2019-03-06)
Fluorinated diiodine alkanes (FDIAs), important industrial intermediates in the synthesis of various perfluorinated compounds, which are distributed widely in wildlife and humans. Recent studies showed that FDIAs had in vitro estrogenic effects. However, to date, little information is available regarding
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![N-[4-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-Heptadecafluorodecyl) benzyloxycarbonyloxy]succinimide ≥97.0% (NMR)](/deepweb/assets/sigmaaldrich/product/structures/232/278/7cb408c4-7b05-4cf3-8e6d-6886bb873b9c/640/7cb408c4-7b05-4cf3-8e6d-6886bb873b9c.png)