448672
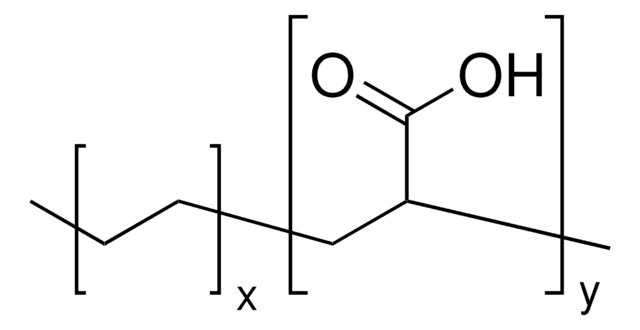
Poly(ethylene-co-acrylic acid)
acrylic acid 15 wt. %
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH2CH2)x[CH2CH(CO2H)]y
Numero CAS:
Numero MDL:
Codice UNSPSC:
12162002
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Composizione
acrylic acid, 15 wt. %
Durezza
≤15 dmm (ASTM D 5, 25 °C)
Viscosità
600 cP(140 °C, Brookfield)(lit.)
Numero di acido
112‑130 mg KOH/g
Temp. transizione
Tm (DSC) 87 °C (at peak)
Densità
0.93 g/mL at 25 °C
Stringa SMILE
C=C.OC(=O)C=C
InChI
1S/C3H4O2.C2H4/c1-2-3(4)5;1-2/h2H,1H2,(H,4,5);1-2H2
QHZOMAXECYYXGP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Processing and performance additive. Promotes crystallization of PET. Assists dispersion of additives in plastics.
Caratteristiche e vantaggi
Forms reversible ionic clusters (crosslinks). Promotes adhesion to various substrates, tougher, more chemically resistant and more transparent than parent acid copolymer.
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Newton C Fawcett et al.
Langmuir : the ACS journal of surfaces and colloids, 20(16), 6651-6657 (2004-07-28)
Yoshimoto et al. [Anal. Chem. 2002, 74, 4306-4309] reported that a quartz crystal microbalance or QCM changed its response to sucrose solutions according to its angle of immersion. The effect was tentatively attributed to gravity-caused stress on the viscous interface
Christoph Meyer et al.
Analytical and bioanalytical chemistry, 382(3), 679-690 (2004-08-04)
Three poly(ethylene-co-acrylic) acid copolymers (-CH(2)CH(2)-)(x)[CH(2)CH(CO(2)H)-](y) with different chain lengths and mass fractions of acrylic acid were covalently immobilized as stationary phases on silica via two variants of spacer molecules (3-aminopropyltriethoxysilane and 3-glycidoxypropyltrimethoxysilane). Different mobilities of the alkyl chains in the
Siri Schauff et al.
Analytical chemistry, 79(21), 8323-8326 (2007-10-09)
The separation process in reversed-phase high-performance liquid chromatography employing C18 phases is mainly due to hydrophobic interactions. The separation of tocopherol isomers, exhibited by the C30 phases, however, is additionally driven by shape selectivity. This phenomenon is investigated by suspended-state
Xiaodi Su et al.
Journal of colloid and interface science, 287(1), 35-42 (2005-05-26)
In this study, we use the quartz crystal microbalance with dissipation monitoring (QCM-D) to study the immobilization of the enzyme horseradish peroxidase (HRP) on poly(ethylene-co-acrylic acid) (PEAA) films. The surface polarity of spin-coated PEAA films was varied by heat treatments
Ning Luo et al.
Colloids and surfaces. B, Biointerfaces, 50(2), 89-96 (2006-06-06)
Poly(ethylene-co-acrylic acid) (EAA) films were reacted with glycine, 12-aminododecanoic acid, aspartic acid, 5-aminoisophthalic acid, ethanolamine, diethylamine, dimethylamine, N-isopropylamine, and dimethylaminoethyleneamine to prepare EAA films with negatively charged, non-charged, hydrophilic, and hydrophobic functionalities. Attenuated total reflectance Fourier transform infrared spectroscopy, differential
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.