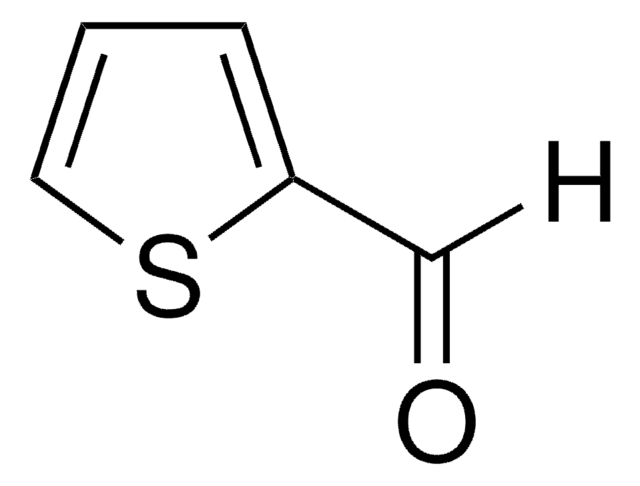
429880
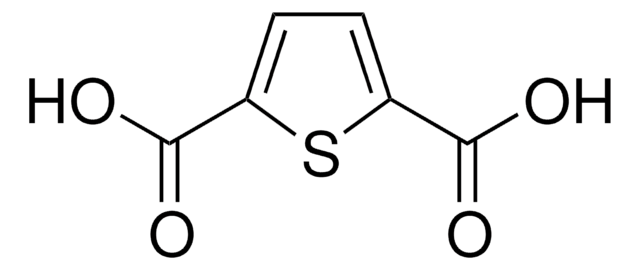
2,5-Thiophenedicarboxaldehyde
99%
Sinonimo/i:
2,5-Diformylthiophene, 2,5-Thienodicarboxaldehyde, 2,5-Thiophenedial, Thiophene-2,5-dialdehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H4O2S
Numero CAS:
Peso molecolare:
140.16
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Punto di fusione
115-117 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
[H]C(=O)c1ccc(s1)C([H])=O
InChI
1S/C6H4O2S/c7-3-5-1-2-6(4-8)9-5/h1-4H
OTMRXENQDSQACG-UHFFFAOYSA-N
Descrizione generale
2,5-Thiophenedicarboxaldehyde can be prepared from 2,5-bis(chloromethyl)thiophene by the application of Kröhnke′s method.
Applicazioni
2,5-Thiophenedicarboxaldehyde may be employed in the following studies:
- Asymmetric synthesis of bis-homoallylic alcohols.
- Synthesis of new symmetrical arylene bisimide derivatives.
- As dialdehyde monomer in the synthesis of silicon-containing poly(p-phenylenevinylene)-related copolymers having uniform p-conjugated segment regulated by organosilicon units.
- Synthesis of 2,5-bis[2-(5-N-isopropylamidino)benzimidazoyl]thiophene hydrochloride.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Xiaoming Li et al.
Frontiers in oncology, 8, 354-354 (2018-10-16)
RNA interference (RNAi) is a biological process through which gene expression can be inhibited by RNA molecules with high selectivity and specificity, providing a promising tool for tumor treatment. Two types of molecules are often applied to inactivate target gene
M Del Poeta et al.
Antimicrobial agents and chemotherapy, 42(10), 2495-2502 (1998-10-03)
Twenty analogues of pentamidine, 7 primary metabolites of pentamidine, and 30 dicationic substituted bis-benzimidazoles were screened for their inhibitory and fungicidal activities against Candida albicans and Cryptococcus neoformans. A majority of the compounds had MICs at which 80% of the
The Preparation of 2, 5-Thiophenedicarboxaldehyde.
Sone T.
Bulletin of the Chemical Society of Japan, 37(8), 1197-1200 (1964)
Marzena Grucela-Zajac et al.
The journal of physical chemistry. C, Nanomaterials and interfaces, 118(24), 13070-13086 (2014-06-27)
New symmetrical arylene bisimide derivatives formed by using electron-donating-electron-accepting systems were synthesized. They consist of a phthalic diimide or naphthalenediimide core and imine linkages and are end-capped with thiophene, bithiophene, and (ethylenedioxy)thiophene units. Moreover, polymers were obtained from a new
Guang-Ming Chen et al.
The Journal of organic chemistry, 64(3), 721-725 (2001-10-25)
Asymmetric reduction of 2,6-diacylpyridines with B-chlorodiisopinocampheylborane provides the corresponding C(2)-symmetric diols in very high de and ee. Asymmetric allylboration of 2,6-pyridinedicarboxaldehyde and 2,5-thiophenedicarboxaldehyde provides the corresponding bis-homoallylic alcohols in very high de and ee. These optically pure diols were converted
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 429880-1G | 4061832107745 |
| 429880-250MG | |
| 429880-5G | 4061832107752 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)