425834
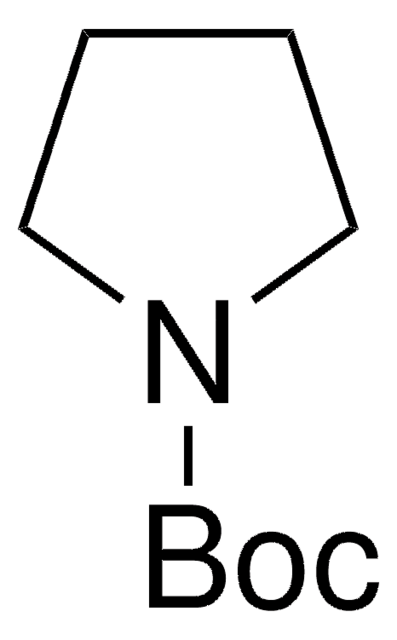
N-Boc-pyrrole
98%
Sinonimo/i:
tert-Butyl 1-pyrrolecarboxylate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H13NO2
Numero CAS:
Peso molecolare:
167.21
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.4685 (lit.)
P. eboll.
91-92 °C/20 mmHg (lit.)
Densità
1 g/mL at 25 °C (lit.)
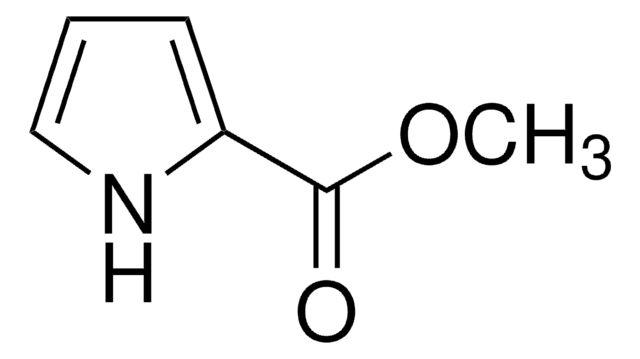
Stringa SMILE
CC(C)(C)OC(=O)n1cccc1
InChI
1S/C9H13NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H,1-3H3
IZPYBIJFRFWRPR-UHFFFAOYSA-N
Descrizione generale
N-Boc-pyrrole is an N-protected pyrrole. It undergoes Diels–Alder reaction with enantiomerically pure allene-1,3-dicarboxylates to form endo-adducts with retention in configurations at two newly generated stereogenic centers. It also undergoes cyclopropanation with methyl phenyldiazoacetate to form both monocyclopropane and dicyclopropane. Its Ir-catalyzed C-H borylation followed by cross coupling with 3-chlorothiophene to form biheterocycle has been reported.
Applicazioni
N-Boc-pyrrole was used in the synthesis of 1-(tert-butoxycarbonyl)-1H-pyrrol-2-ylboronic acid by treating with n-BuLi and subsequent reaction with trimethyl borate.
It may be used as starting material in the synthesis of the following:
It may be used as starting material in the synthesis of the following:
- tropane drivatives
- N-boc-2-(4-methoxyphenyl)pyrrole
- N-boc-pyrrol-2-ylboronic acid
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
167.0 °F - closed cup
Punto d’infiammabilità (°C)
75 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Synthetic approaches to enantiomerically pure 8-azabicyclo [3.2. 1] octane derivatives.
Pollini GP, et al.
Chemical Reviews, 106(6), 2434-2454 (2006)
Huw M L Davies et al.
Chemical Society reviews, 38(11), 3061-3071 (2009-10-23)
The metal catalyzed reactions of diazo compounds have been broadly used in organic synthesis. The resulting metal-carbenoid intermediates are capable of undergoing a range of unconventional reactions, and due to their high energy, they are ideal for initiating cascade sequences
Recent progress in the synthesis of five-membered heterocycle boronic acids and esters.
Primas N, et al.
Tetrahedron, 66(41), 8121-8136 (2010)
Nikola Basarić et al.
Organic & biomolecular chemistry, 3(15), 2755-2761 (2005-07-21)
Two fluorescent off-on Ca2+ indicators based on APTRA (o-aminophenol-N,N,O-triacetic acid) as low-affinity ligand for Ca2+ and BODIPY(4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) as a fluorophore were synthesized. The new BODIPY-APTRA compounds absorb in the visible spectrum, with absorption maxima from 505 nm to 570 nm
CuO/SiO2 as a simple, effective and recoverable catalyst for alkylation of indole derivatives with diazo compounds.
Fraile JM, et al.
Organic & Biomolecular Chemistry, 11(26), 4327-4332 (2013)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.