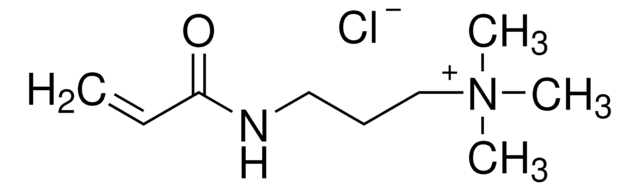
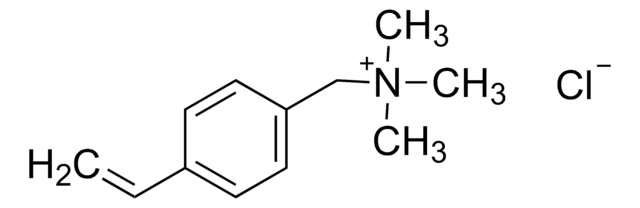
403245
(3-Carboxypropyl)trimethylammonium chloride
technical grade
Sinonimo/i:
γ-Butyrobetaine hydrochloride, Deoxycarnitine hydrochloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C7H16NO2Cl
Numero CAS:
Peso molecolare:
181.66
Numero MDL:
Codice UNSPSC:
12352116
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Grado
technical grade
Punto di fusione
220 °C (dec.) (lit.)
Stringa SMILE
[Cl-].C[N+](C)(C)CCCC(O)=O
InChI
1S/C7H15NO2.ClH/c1-8(2,3)6-4-5-7(9)10;/h4-6H2,1-3H3;1H
GNRKTORAJTTYIW-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
J S Serrano et al.
Methods and findings in experimental and clinical pharmacology, 5(4), 251-254 (1983-01-01)
The effects of GABA, its quaternary derivative N-trimethyl GABA and quinidine have been studied in a model of ventricular automaticity induced in the isolated right ventricle of the rat. GABA shows low anti-automatic activity. N-trimethyl GABA and quinidine were, however
Frédéric M Vaz et al.
The Biochemical journal, 361(Pt 3), 417-429 (2002-01-23)
Carnitine is indispensable for energy metabolism, since it enables activated fatty acids to enter the mitochondria, where they are broken down via beta-oxidation. Carnitine is probably present in all animal species, and in numerous micro-organisms and plants. In mammals, carnitine
L M Henderson et al.
Federation proceedings, 41(12), 2843-2847 (1982-10-01)
The biosynthesis of carnitine proceeds from trimethyllysine (TML) by beta-hydroxylation by a liver or kidney mitochondrial enzyme, which requires oxygen, alpha-ketoglutarate, ferrous iron, and ascorbate. This dioxygenase is rapidly inactivated by preincubation with Fe2+, but not Fe3+. The evidence suggests
P J Nelson et al.
Biochimica et biophysica acta, 672(1), 123-127 (1981-01-07)
The effects of ascorbate deficiency on carnitine biosynthesis was investigated in young male guinea pigs. Liver and kidney carnitine levels were not affected by the deficiency, but scorbutic animals had 50% less carnitine in heart and skeletal muscle than control
C J Gross et al.
Biochimica et biophysica acta, 886(3), 425-433 (1986-05-29)
Uptake and metabolism of L-carnitine, D-carnitine and acetyl-L-carnitine were studied utilizing isolated guinea-pig enterocytes. Uptake of the D- and L-isomers of carnitine was temperature dependent. Uptake of L-[14C]carnitine by jejunal cells was sodium dependent since replacement by lithium, potassium or
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[2-(Acryloyloxy)ethyl]trimethylammonium chloride solution 80 wt. % in H2O, contains 600 ppm monomethyl ether hydroquinone as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/393/326/f7e19585-5431-4220-81b5-f458de6d63d0/640/f7e19585-5431-4220-81b5-f458de6d63d0.png)
![[3-(Methacryloylamino)propyl]trimethylammonium chloride solution 50 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/189/736/089bc8ae-2a98-416d-9f9a-a0a510b6b828/640/089bc8ae-2a98-416d-9f9a-a0a510b6b828.png)
![[2-(Methacryloyloxy)ethyl]trimethylammonium chloride solution 75 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/316/612/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe/640/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe.png)