392391
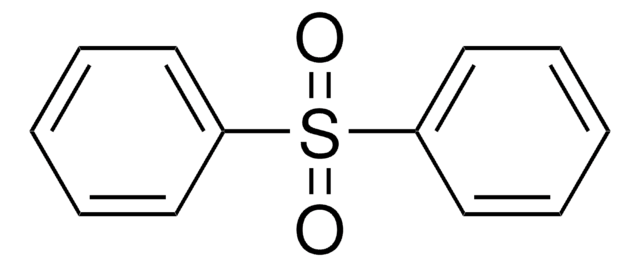
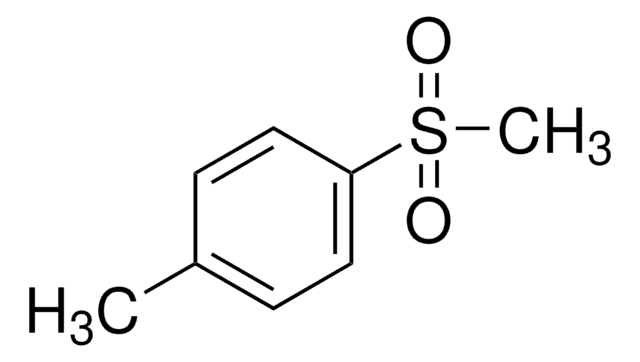
Di-p-tolyl sulfone
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH3C6H4)2SO2
Numero CAS:
Peso molecolare:
246.32
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Forma fisica
solid
Punto di fusione
158-160 °C (lit.)
Solubilità
chloroform: soluble 25 mg/mL, clear, colorless to yellow
Gruppo funzionale
sulfone
Stringa SMILE
Cc1ccc(cc1)S(=O)(=O)c2ccc(C)cc2
InChI
1S/C14H14O2S/c1-11-3-7-13(8-4-11)17(15,16)14-9-5-12(2)6-10-14/h3-10H,1-2H3
WEAYCYAIVOIUMG-UHFFFAOYSA-N
Descrizione generale
Di-p-tolyl sulfone is a di-p-substituted diaryl sulfone. Its synthesis by the sulfonylation of toluene with p-toluenesulfonic acid (TsOH) in the presence of polystyrene supported aluminium triflate (Ps-Al(OTf)3) catalyst has been reported along with its NMR and IR spectra. The gas-phase heats of formation of di-p-tolyl sulfone has been studied. The kinetics and thermodynamics of sulphuric acid assisted cleavage of di-p-tolyl sulfone has been invesitigated.
Applicazioni
Di-p-tolyl sulfone may be used in the synthesis of isomeric p-tolylpyridines (α , β and γ ) by photochemical decomposition.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Para tolylation of pyridine by photolysis of di-p-tolyl sulfone and related compounds.
Nakabayashi T, et al.
Bulletin of the Chemical Society of Japan, 50(9), 2491-2492 (1977)
Studies in the thermochemistry of sulphones. Part 6.-Heats of combustion, fusion, vaporization and atomization of six aromatic and two allylic sulphones.
Mackle H and O'Hare PAG.
Transactions of the Faraday Society, 57, 1521-1526 (1961)
Polystyrene Supported Al (OTf)3: a Stable, Efficient, Selective, and Reusable Catalyst for Sulfonylation of Arenes with Sulfonic Acids.
Boroujeni, KP.
Bull. Korean Chem. Soc., 31(7), 1887-1890 (2010)
Kinetics and thermodynamics of sulfuric acid-mediated cleavage of substituted diaryl sulfones.
Ward RS, et al.
Journal of Surfactants and Detergents, 4(2), 185-190 (2001)
Kazumasa Okamoto et al.
Scientific reports, 10(1), 19823-19823 (2020-11-15)
Dimer radical ions of aromatic molecules in which excess charge is localized in a pair of rings have been extensively investigated. While dimer radical cations of aromatics have been previously produced in the condensed phase, the number of molecules that
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.