379387
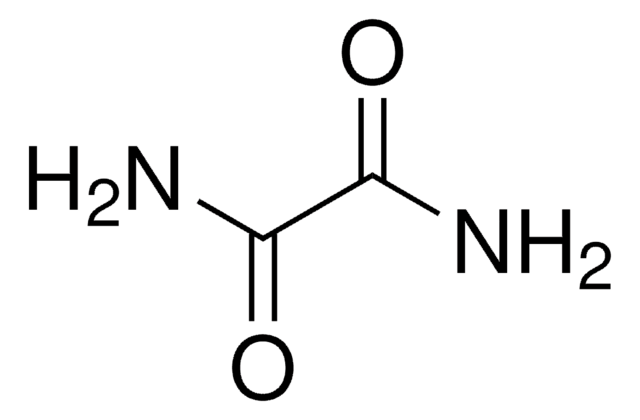
Dithiooxamide
97%
Sinonimo/i:
Dithiooxalic diamide, Rubeanic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NH2CSCSNH2
Numero CAS:
Peso molecolare:
120.20
Beilstein:
605577
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder
Punto di fusione
≥300 °C (lit.)
Solubilità
ethanol: soluble 40 mg/10 mL, clear, red
Gruppo funzionale
amine
Stringa SMILE
NC(=S)C(N)=S
InChI
1S/C2H4N2S2/c3-1(5)2(4)6/h(H2,3,5)(H2,4,6)
OAEGRYMCJYIXQT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Dithiooxamide is reported to form complexes with Ni(II).
Applicazioni
Dithiooxamide may be used in the following studies:
- Synthesis of thiazolothiazole-linked porous organic polymers under solvothermal conditions.
- As modifier to prepare the modified glassy carbon electrode, used to investigate the electrochemical properties of quercetin, an important flavonoid derivative.
- Synthesis of new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF), used in separation and concentration of silver ions.
- Synthesis of N,N′-disubstituted dithiooxamides.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Xiang Zhu et al.
Chemical communications (Cambridge, England), 50(95), 15055-15058 (2014-10-21)
Thiazolothiazole-linked porous organic polymers have been synthesized from a facile catalyst-free condensation reaction between aldehydes and dithiooxamide under solvothermal conditions. The resultant porous frameworks exhibit a highly selective uptake of CO2 over N2 under ambient conditions.
Preparation of Dithiooxamide Derivatives.
Hurd RN, et al.
The Journal of Organic Chemistry, 26(10), 3980-3987 (1961)
Nickel (II) complexes with dithiooxamide, N, N'-di-methyl-and N, N'-di-hydroxyethyl-dithiooxamide.
Peyronel G, et al.
Inorgorganica Chimica Acta, 5, 627-633 (1971)
Ayşen Demir Mülazımoğlu et al.
Sensors (Basel, Switzerland), 12(4), 3916-3928 (2012-06-06)
Electrochemical oxidation of quercetin, as an important biological molecule, has been studied in non-aqueous media using cyclic voltammetry, electrochemical impedance spectroscopy and scanning electron microscopy. To investigate the electrochemical properties of quercetin, an important flavonoid derivative, on a different surface
Zeliyha Celik et al.
Journal of hazardous materials, 174(1-3), 556-562 (2009-10-13)
In this study, a new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF) has been synthesized by the reaction of dithiooxamide and formaldehyde. Also a well-known chelating resin of thiourea (thiooxamide)-formaldehyde (TUF) has been prepared by the reaction of thiourea and
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 379387-25G | 4061831837056 |
| 379387-5G | 4061831837063 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.