328367
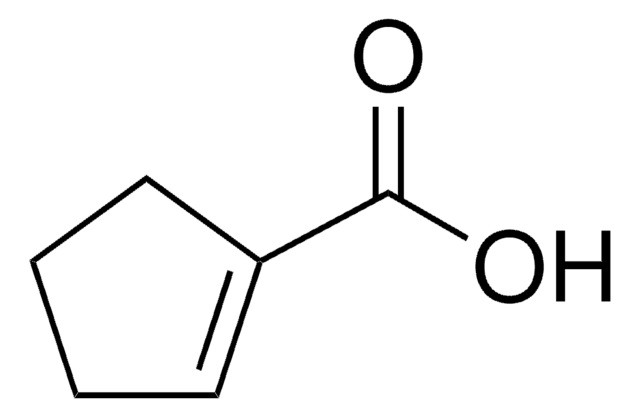
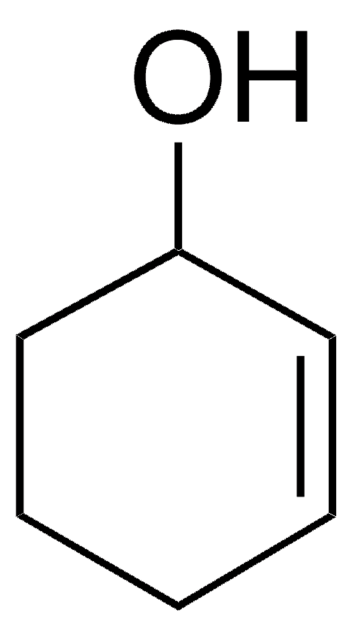
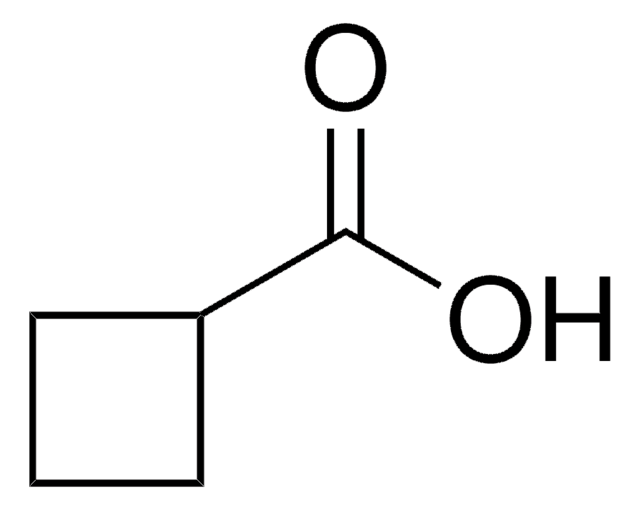
1-Cyclohexene-1-carboxylic acid
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H9CO2H
Numero CAS:
Peso molecolare:
126.15
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
P. ebollizione
133-135 °C/14 mmHg (lit.)
Punto di fusione
35-39 °C (lit.)
Densità
1.101 g/mL at 25 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
OC(=O)C1=CCCCC1
InChI
1S/C7H10O2/c8-7(9)6-4-2-1-3-5-6/h4H,1-3,5H2,(H,8,9)
NMEZJSDUZQOPFE-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
1-Cyclohexene-1-carboxylic acid was identified as intermediate during the anaerobic decomposition of benzoic acid by a methanogenic consortium.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
C L Keith et al.
Archives of microbiology, 118(2), 173-176 (1978-08-01)
A possible pathway for the anaerobic utilization of benzoic acid by a methanogenic consortium is suggested. Cyclohexane carboxylic acid and 1-cyclohexene-1-carboxylic acid have been identified as intermediates before ring rupture. Suprisingly, 3-cyclohexene-1-carboxylic acid interferes with utilization of other cyclic acids.
Mariana G de Oliveira et al.
American journal of physiology. Renal physiology, 315(3), F460-F468 (2018-05-03)
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a chronic inflammatory disease without consistently effective treatment. We investigate the role of toll-like receptor 4 (TLR4) on voiding dysfunction and inflammation in the cyclophosphamide (CYP)-induced mouse cystitis. Male C57BL/6 [wild-type, (WT)] and/or TLR4
K A Reynolds et al.
Journal of bacteriology, 174(12), 3850-3854 (1992-06-01)
A novel NADPH-dependent enoyl reductase, catalyzing the conversion of 1-cyclohexenylcarbonyl coenzyme A (1-cyclohexenylcarbonyl-CoA) to cyclohexylcarbonyl-CoA, was purified to homogeneity from Streptomyces collinus. This enzyme, a dimer with subunits of identical M(r) (36,000), exhibits a Km of 1.5 +/- 0.3 microM
Xin Xie et al.
Carbohydrate polymers, 225, 115223-115223 (2019-09-16)
A polysaccharide isolated from Strongylocentrotus nudus eggs (SEP) reportedly displays immune activity in vivo. Here, its effect and underlying mechanism in the treatment of pancreatic cancer were investigated. SEP obviously inhibited pancreatic cancer growth by activating NK cells in vitro/vivo
M S Elshahed et al.
Applied and environmental microbiology, 67(4), 1728-1738 (2001-04-03)
The metabolism of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate by "Syntrophus aciditrophicus" in cocultures with hydrogen-using microorganisms was studied. Cyclohexane carboxylate, cyclohex-1-ene carboxylate, pimelate, and glutarate (or their coenzyme A [CoA] derivatives) transiently accumulated during growth with benzoate. Identification was
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 328367-5G | 4061836676650 |
| 328367-1G | 4061826717448 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.