213497
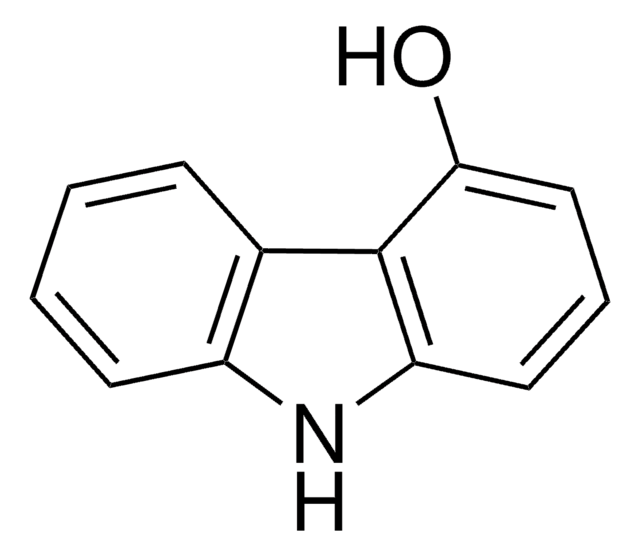
2-Hydroxycarbazole
97%
Sinonimo/i:
2-Carbazolol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
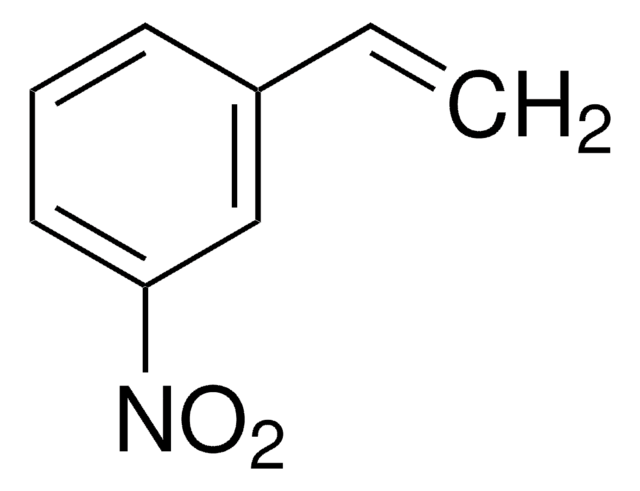
C12H9NO
Numero CAS:
Peso molecolare:
183.21
Beilstein:
135859
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
270-273 °C (lit.)
Stringa SMILE
Oc1ccc2c(c1)[nH]c3ccccc23
InChI
1S/C12H9NO/c14-8-5-6-10-9-3-1-2-4-11(9)13-12(10)7-8/h1-7,13-14H
GWPGDZPXOZATKL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2-Hydroxycarbazole is a compound structurally related to the Ca2+-mobilizing marine toxin, 9-methyl-7-bromoeudistomin. Room temperature electronic absorption and fluorescence spectra of 2-hydroxycarbazole has been studied in concentrated aqueous potassium hydroxide solutions. It undergoes chemoselective N-alkylation using NaH as a base in a THF-DMF solvent system.
Applicazioni
2-Hydroxycarbazole was used in the synthesis of isochromene fused carbazol, (4aS,13bR)-2,5,5-trimethyl-3,4,4a,5,8,13b-hexahydroisochromeno[3,4-b]carbazole.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Tamanna Mallick et al.
Colloids and surfaces. B, Biointerfaces, 172, 440-450 (2018-09-10)
Six structurally different carbazoles (1-6) were explored as the green reducing agents for the synthesis of the fluorescent Au nanoparticles with tailor-made morphology in anionic (sodium dodecyl sulphate, SDS), cationic (cetyltrimethylammonium bromide, CTAB) and neutral (polyvinylpyrrolidone, PVP) micelle medium. Structure
Nguyen Manh Cuong et al.
Natural product communications, 4(7), 921-924 (2009-09-08)
The first synthesis of isochromene fused carbazols, (4aS, 13bR)-2,5,5-trimethyl-3,4,4a,5,8,13b-hexahydroisochromeno[3,4-b]carbazole (2) and its epi-isomer 3 by condensation of citral and 2-hydroxycarbazole using Ti(OEt)4 and MeAlC12 as catalysts is described.
S C Tovey et al.
European journal of pharmacology, 354(2-3), 245-251 (1998-10-01)
2-Hydroxycarbazole was shown to induce Ca2+ release from skeletal muscle and cardiac muscle sarcoplasmic reticulum at concentrations between 100-500 microM. This release was blocked by both 1 mM tetracaine and 30 microM ruthenium red which inhibit the ryanodine receptor or
J Doutheil et al.
Cell calcium, 25(6), 419-428 (1999-12-01)
In the physiological state, protein synthesis is controlled by calcium homeostasis in the endoplasmic reticulum (ER). Recently, evidence has been presented that dividing cells can adapt to an irreversible inhibition of the ER calcium pump (SERCA), although the mechanisms underlying
Chemoselective N-alkylation of 2-hydroxycarbazole as a model for the synthesis of N-substituted pyrrole derivatives containing acidic functions.
Albanese D, et al.
Tetrahedron, 51(19), 5681-5688 (1995)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.