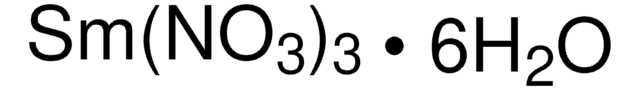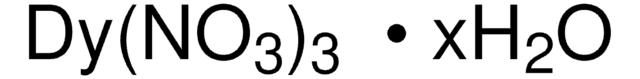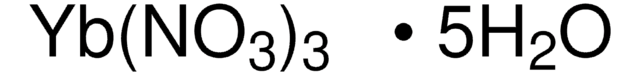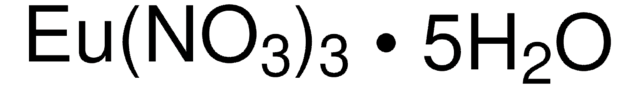205133
Praseodymium(III) nitrate hexahydrate
99.9% trace metals basis
Sinonimo/i:
Praseodymium trinitrate hexahydrate
About This Item
Prodotti consigliati
Saggio
99.9% trace metals basis
Forma fisica
crystalline
Impiego in reazioni chimiche
reagent type: catalyst
core: praseodymium
Impurezze
≤2000 ppm Trace Metal Analysis
Stringa SMILE
O.O.O.O.O.O.[Pr+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/3NO3.6H2O.Pr/c3*2-1(3)4;;;;;;;/h;;;6*1H2;/q3*-1;;;;;;;+3
LXXCECZPOWZKLC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- A dopant to fabricate dye-sensitized solar cells. The addition of rare earth enhances the power conversion efficiency of solar cells by narrowing the band gap of photoanode materials.
- A precursor to synthesize high entropy lanthanide oxysulfides ( wide band gap semiconductors).
- To synthesize functionalized UV-emitting nanocomposite for photodynamic cancer therapy.
- To fabricate Pr-doped MoO3 thinfilms for gas sensing applications.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2
Codice della classe di stoccaggio
5.1B - Oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 2
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.