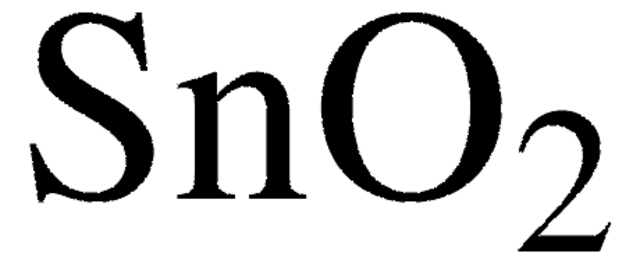199478
Germanium(IV) oxide
(crystalline powder), 99.998% trace metals basis
Sinonimo/i:
Germanium dioxide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
GeO2
Numero CAS:
Peso molecolare:
104.64
Numero CE:
Numero MDL:
Codice UNSPSC:
12352303
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Livello qualitativo
Saggio
99.998% trace metals basis
Stato
(crystalline powder)
Punto di fusione
>400 °C (lit.)
Densità
4.23 g/cm3
applicazioni
battery manufacturing
Stringa SMILE
O=[Ge]=O
InChI
1S/GeO2/c2-1-3
YBMRDBCBODYGJE-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Germanium(IV) oxide (GeO₂) is a semiconductor material known for its notable optical properties and high refractive index. It finds extensive use in various applications within electronics and optics. This compound is slightly soluble in water and acts as a catalyst in industrial processes. Additionally, GeO₂ is employed in the development of sensors for gas detection and other chemical species, utilizing its semiconductor characteristics to enhance sensitivity and response times.
Applicazioni
Germanium(IV) oxide can be used:
- To prepare glasses for nuclear radiation shielding and ultra-broadband amplification. For example, germanium and bismuth oxide glasses.
- As a precursor to synthesize GeO2/TiO2 nanocomposites that can be used for the photocatalysis of organic pollutants.
- As an anode material for lithium-ion batteries due to its high lithium storage capacity, thermostability, and large volume expansion during the charge–discharge process.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Zita Oláh et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 149, 75-82 (2019-04-29)
The radiochemical separation of n.c.a. arsenic on its own or for radio-labelling purposes usually involves the issue of reducing arsenic(V). Numerous approaches for reducing pentavalent arsenic have been examined. A novel HPLC method has also been presented for accessing the
P. M. Lambert
Inorganic chemistry, 37(6), 1352-1357 (2001-10-24)
The gel chemistry of germanium is explored through the formation and composition of a hydrous metal oxide precursor gel used in the preparation of the HfGeO(4) and HfGeO(4):Ti X-ray phosphors. The enhanced solubility of hexagonal GeO(2) in dilute ammoniacal solutions
Xinguo Hong et al.
The Review of scientific instruments, 80(7), 073908-073908 (2009-08-07)
We describe an approach for acquiring high quality x-ray absorption fine structure (XAFS) spectroscopy spectra with wide energy range at high pressure using diamond anvil cell (DAC). Overcoming the serious interference of diamond Bragg peaks is essential for combining XAFS
Sarah L Sewell et al.
Dalton transactions (Cambridge, England : 2003), (29)(29), 3857-3865 (2008-07-17)
Biomimetic synthesis is emerging as an advantageous alternative to the harsh synthetic conditions traditionally used in metal oxide syntheses techniques. Silaffins, proteins from the C. fusiformis diatom, form silica in an aqueous environment under benign conditions. Amine terminated PAMAM and
E A Anashkina et al.
Optics express, 20(24), 27102-27107 (2012-11-29)
We report generation of femtosecond optical pulses tunable in the 1.6-2.5 μm range using GeO2-doped core silica-cladding fibers. Optical solitons with a duration of 80-160 fs have been measured by the FROG technique in the 2-2.3 μm range. To the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.