184144
3,5-Dinitrobenzyl alcohol
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(O2N)2C6H3CH2OH
Numero CAS:
Peso molecolare:
198.13
Beilstein:
2054388
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
88-91 °C (lit.)
Gruppo funzionale
hydroxyl
nitro
Stringa SMILE
OCc1cc(cc(c1)[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H6N2O5/c10-4-5-1-6(8(11)12)3-7(2-5)9(13)14/h1-3,10H,4H2
GPHYIQCSMDYRGJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
3,5-Dinitrobenzyl alcohol on reaction with p-toluenesulphonyl chloride yields 3,5-dinitrobenzyl p-toluenesulphonate.
Applicazioni
3,5-Dinitrobenzyl alcohol was used in the synthesis of 3,5-bis((bezoxycarbonyl)imino)benzyl alcohol.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Ludmila Eisner et al.
Sensors (Basel, Switzerland), 19(18) (2019-09-13)
A sensor for trinitrotoluene (TNT) detection was developed by using a combination of optical micro-ring technology and a receptor coating based on molecularly imprinted sol-gel layers. Two techniques for deposition of receptor layers were compared: Airbrush technology and electrospray ionization.
K Funazo et al.
Journal of chromatography, 481, 211-219 (1989-11-03)
New UV-labelling agents have been synthesized, which are designed to convert monocarboxylic acids into their highly UV-absorbing derivatives for enhancement of the sensitivities of UV detection in high-performance liquid chromatography. The reagents are p-nitrobenzyl, 3,5-dinitrobenzyl and 2-(phthalimino)ethyl p-toluenesulphonates. Each has
Synthesis and characterization of hyperbranched polyurethanes prepared from blocked isocyanate monomers by step-growth polymerization.
Spindler R and Frechet JMJ.
Macromolecules, 26(18), 4809-4813 (1993)
Evon Powell et al.
International journal of nanomedicine, 2(3), 449-459 (2007-11-21)
The interaction of the important but often overdosed local anesthetic bupivacaine, its structural analogs 2,6-dimethylaniline, and N-methyl-2,6-dimethylacetanilide, and cocaine, with several electron deficient aromatic moieties were studied primarily by proton NMR and UV-visible spectroscopy. In solution, the anesthetic, its analogs
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.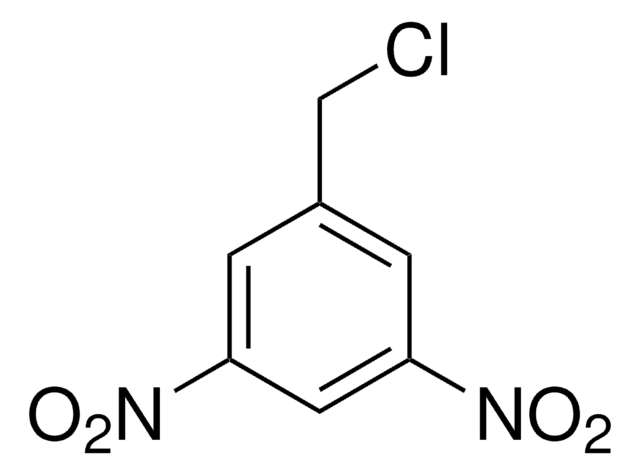
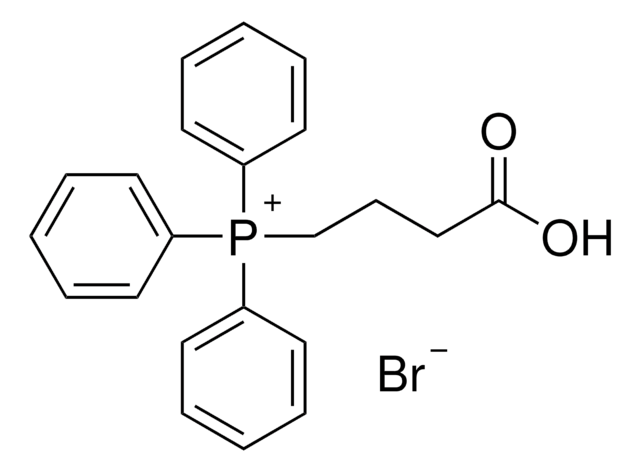
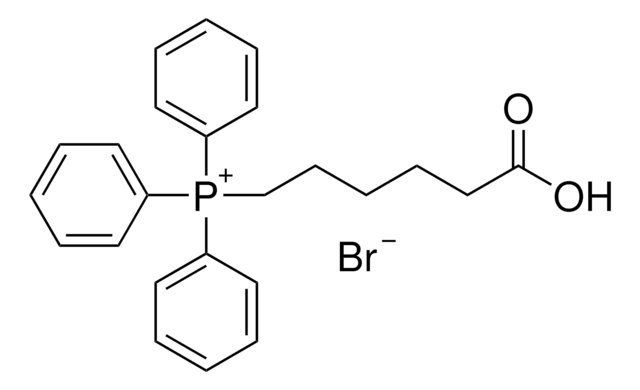
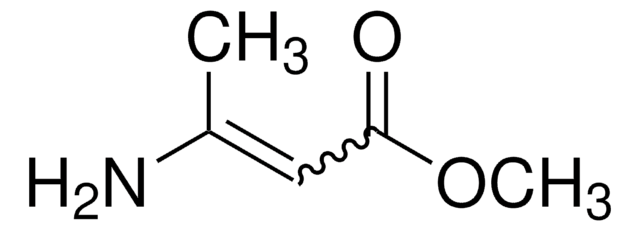

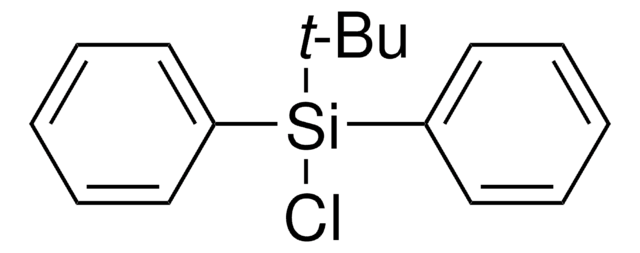
![1,8-diazabiciclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)