15408
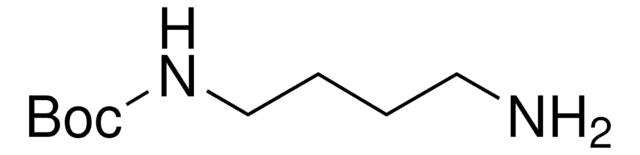
N-Boc-1,3-propanediamine
≥97.0% (GC/NT)
Sinonimo/i:
N-Boc-1,3-diaminopropane, tert-Butyl N-(3-aminopropyl)carbamate
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥97.0% (GC/NT)
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Indice di rifrazione
n20/D 1.454 (lit.)
n20/D 1.459
P. ebollizione
203 °C (lit.)
Punto di fusione
22 °C (lit.)
Densità
0.998 g/mL at 20 °C (lit.)
Gruppo funzionale
Boc
amine
Stringa SMILE
NCCCNC(OC(C)(C)C)=O
InChI
1S/C8H18N2O2/c1-8(2,3)12-7(11)10-6-4-5-9/h4-6,9H2,1-3H3,(H,10,11)
POHWAQLZBIMPRN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Tackling vancomycin-resistant bacteria with ′lipophilic–vancomycin–carbohydrate conjugates′: This study discusses the synthesis of derivatives using N-Boc-1,3-propanediamine to develop new antibacterial agents targeting resistant bacterial strains (Yarlagadda et al., 2015).
- Sulfonamides differing in the alkylamino substituent length–Synthesis, electrochemical characteristic, acid-base profile and complexation properties: The study involves N-Boc-1,3-propanediamine in the synthesis of novel sulfonamide derivatives with potential biochemical applications (Ciesielska et al., 2022).
- Direct α-alkylation of primary aliphatic amines enabled by CO2 and electrostatics: Research demonstrating selective α-alkylation of N-Boc-1,3-propanediamine, highlighting a novel method in organic synthesis (Ye et al., 2018).
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
228.2 °F - closed cup
Punto d’infiammabilità (°C)
109 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.