142824
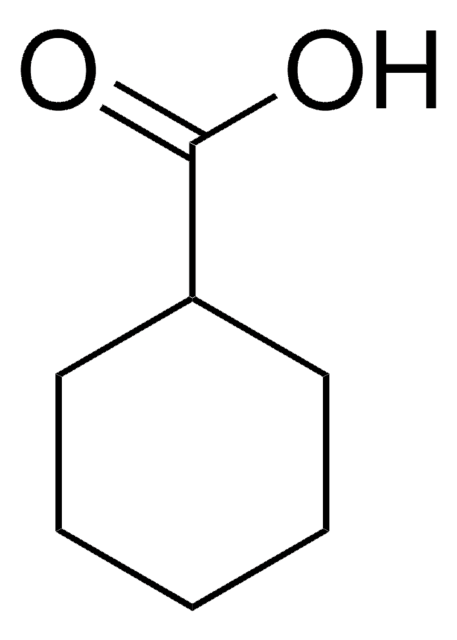
1-Methyl-1-cyclohexanecarboxylic acid
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
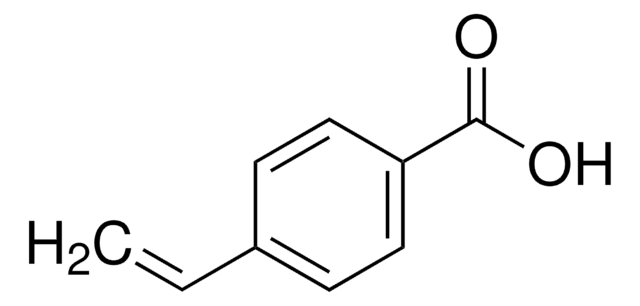
Formula condensata:
CH3C6H10CO2H
Numero CAS:
Peso molecolare:
142.20
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
solid
P. ebollizione
234 °C (lit.)
Punto di fusione
36-39 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
CC1(CCCCC1)C(O)=O
InChI
1S/C8H14O2/c1-8(7(9)10)5-3-2-4-6-8/h2-6H2,1H3,(H,9,10)
REHQLKUNRPCYEW-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
1-Methyl-1-cyclohexanecarboxylic acid is the structural analog of valproic acid and its pharmacokinetic action has been studied in female Sprague-Dawley rats.
Applicazioni
1-Methyl-1-cyclohexanecarboxylic acid was used as internal standard during the determination of valproic acid metabolites.
Azioni biochim/fisiol
1-Methyl-1-cyclohexanecarboxylic acid is an anticonvulsant drug and causes maturation of murine neuroblastoma cells in vitro.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
213.8 °F - closed cup
Punto d’infiammabilità (°C)
101.00 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
J C Larcher et al.
Experimental cell research, 203(1), 72-79 (1992-11-01)
Adriamycin, an anticancer agent acting on topoisomerase II, promotes the arrest of cell division and neurite extension in a "neurite-minus" murine neuroblastoma cell line, N1A-103. This morphological differentiation is accompanied by a blockade in the S phase of the cell
J C Larcher et al.
Oncogene, 6(4), 633-638 (1991-04-01)
Using clones N1E-115 and N1A-103 from mouse neuroblastoma C1300, a comparative analysis of c- and N-myc gene expression was undertaken both in proliferating cells and in cultures exposed to conditions which induce differentiation. Under the latter conditions, while N1E-115 cells
A J Sadeque et al.
The Journal of pharmacology and experimental therapeutics, 283(2), 698-703 (1997-11-14)
Cytochrome P450-dependent desaturation of the anticonvulsant drug valproic acid (VPA) results in formation of the hepatotoxin, 4-ene-VPA. Polytherapy with other anticonvulsants which are known P450 inducers increases the flux through this bioactivation pathway. The aim of the present study was
S A Fischkoff et al.
Journal of biological response modifiers, 3(2), 132-137 (1984-01-01)
The anticonvulsant drug 1-methyl-1-cyclohexanecarboxylic acid ( MCCA ) has been shown to cause maturation of murine neuroblastoma cells in vitro at concentrations that are pharmacologically achievable. HL-60 human promyelocytic leukemia cells cultured with this drug underwent a dose-dependent decrease in
J L Vayssiere et al.
Biochemical and biophysical research communications, 140(3), 789-796 (1986-11-14)
CCA, a potent neuroblastoma differentiation inducer, was shown by oxygraphic measurements to reduce significantly the O2 consumption of whole neuroblastoma cells as of mitochondria purified from neuroblastoma or mouse cortex. The effect of CCA on the respiration was compared to
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 142824-1G | |
| 142824-10G | 4061838734495 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.