133299
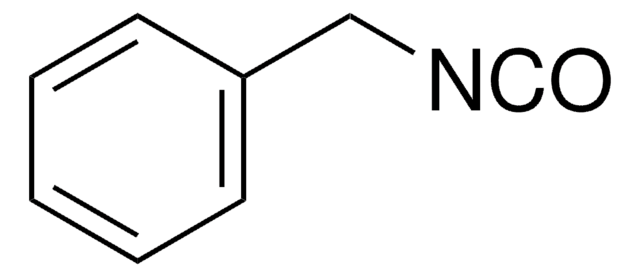
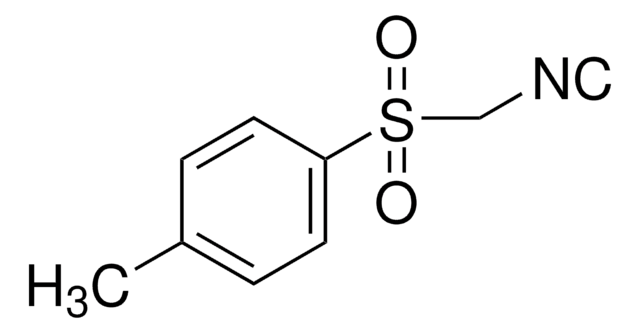
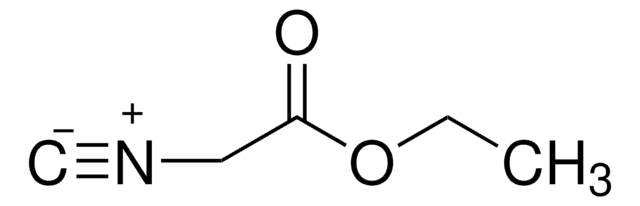
Benzyl isocyanide
98%
Sinonimo/i:
(Isocyanomethyl)benzene, Benzyl isonitrile
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5CH2NC
Numero CAS:
Peso molecolare:
117.15
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Indice di rifrazione
n20/D 1.521 (lit.)
P. ebollizione
105-106 °C/75 mmHg (lit.)
Densità
0.962 g/mL at 25 °C (lit.)
Gruppo funzionale
amine
isonitrile
phenyl
Temperatura di conservazione
−20°C
Stringa SMILE
[C-]#[N+]Cc1ccccc1
InChI
1S/C8H7N/c1-9-7-8-5-3-2-4-6-8/h2-6H,7H2
RIWNFZUWWRVGEU-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Benzyl isocyanide forms phosphaalkene-containing complexes.
Applicazioni
Benzyl isocyanide was used in the synthesis of Ru(II) complexes containing hydrazine and benzyl isocyanide ligands. It was used in a three-component coupling process leading to O- and N-arylamides.
Altre note
May darken in storage
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
174.2 °F - closed cup
Punto d’infiammabilità (°C)
79 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Samantha N MacMillan et al.
Chemical communications (Cambridge, England), (40)(40), 4172-4174 (2007-10-11)
Reaction of (N(3)N)ZrPHPh (N(3)N=N(CH(2)CH(2)NSiMe(3))(3)(3-)) with PhCH(2)N[triple bond]C affords the 1,1-insertion product (N(3)N)Zr[C(PHPh)=NCH(2)Ph], which thermally rearranges to the phosphaalkene-containing complex, (N(3)N)Zr[N(CH(2)Ph)C(H)=PPh].
Laurent El Kaïm et al.
The Journal of organic chemistry, 72(11), 4169-4180 (2007-04-26)
The use of Smiles rearrangement in Ugi- and Passerini-type couplings with electron-deficient phenols allows very straightforward multicomponent formation of O-aryl- and N-arylamides. Best yields were observed with the highly activated o- and p-nitrophenols, salicylic derivatives giving adducts in lower yields.
Synthesis and X-ray studies of ruthenium (II) complexes containing hydrazine and benzyl isocyanide ligands.
Owalude SO, et al.
Bulletin of the Chemical Society of Ethiopia, 27(3), 405-411 (2013)
Zeinab Faghih et al.
Iranian journal of pharmaceutical research : IJPR, 19(3), 134-143 (2021-03-09)
The complex [(PhCH2NC)AuCl], 1, was prepared by the reaction of [(Me2S)AuCl], A, with an equimolar amount of benzyl isocyanide (PhCH2NC) ligand. Through a salt metathesis reaction, the chloride ligand in 1 was replaced by potassium benzothiazole-2-thiolate (Kbt) and potassium benzoimidazole-2-thiolate
Ben J Tickner et al.
Chemical science, 10(20), 5235-5245 (2019-06-14)
We report the formation of a series of novel [Ir(H)2(IMes)(α-13C2-carboxyimine)L] complexes in which the identity of the coligand L is varied. When examined with para-hydrogen, complexes in which L is benzylamine or phenethylamine show significant 1H hydride and 13C2 imine
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 133299-250MG | |
| 133299-5G | 4061838729422 |
| 133299-1G | 4061838729415 |
| 133299-1KG |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.