109789
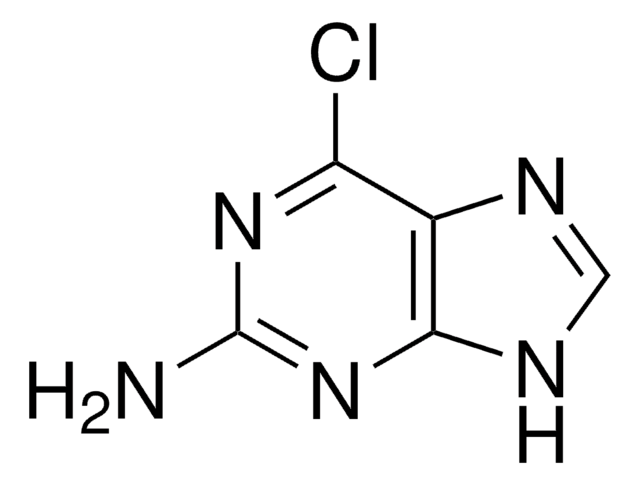
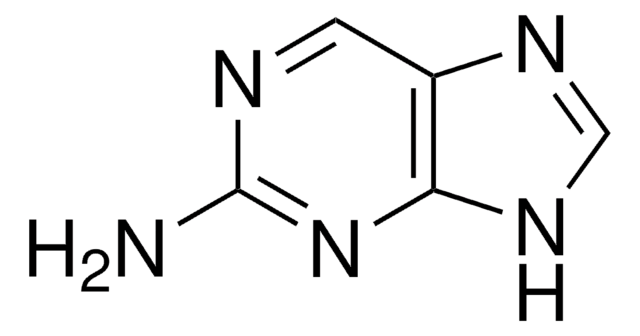
2-Amino-6-chloropurine
≥99%
Sinonimo/i:
6-Chloro-2-purinamine, 6-Chloroguanine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H4ClN5
Numero CAS:
Peso molecolare:
169.57
Beilstein:
9626
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥99%
Punto di fusione
>300 °C (lit.)
Gruppo funzionale
chloro
Stringa SMILE
Nc1nc(Cl)c2nc[nH]c2n1
InChI
1S/C5H4ClN5/c6-3-2-4(9-1-8-2)11-5(7)10-3/h1H,(H3,7,8,9,10,11)
RYYIULNRIVUMTQ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
2-Amino-6-chloropurine has been used in the enzymatic production of DAPdR and 2-amino-6-chloropurine-2,-deoxyriboside (ACPdR).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
K Kim et al.
Combinatorial chemistry & high throughput screening, 3(2), 125-129 (2000-05-02)
A new application of solid-supported reagents was developed to separate the alkylated N7/N9 regioisomers derived from commercially available 2-amino-6-chloropurine. Simple filtration through an alumina/H+ pad or scavenging by AG/Dowex-50W-X8 resin provides diverse N9 regioisomers selectively in moderate yields with high
L L Bennett et al.
Biochemical pharmacology, 33(2), 261-271 (1984-01-15)
2-Amino-6-chloro-1-deazapurine is of interest as a purine analog with demonstrated in vivo activity against mouse leukemia L1210. That the active form of this agent is a nucleotide and that the nucleotide is formed by the action of hypoxanthine (guanine) phosphoribosyltransferase
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 340-351 (2012-06-19)
Two purine tautomers of 2-amino-6-chloropurine (ACP), in labeled as N(9)H(10) and N(7)H(10), were investigated by vibrational spectroscopy and quantum chemical method. The FT-IR and FT-Raman spectra of ACP have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively.
Lak Shin Jeong et al.
Nucleosides, nucleotides & nucleic acids, 26(6-7), 721-724 (2007-12-11)
Novel apio carbocyclic nucleosides 18-21 were asymmetrically synthesized as potential antiviral and antitumor agent, starting from D-ribose employing aldol reaction, RCM reaction and Mitsunobu reaction as key reactions.
Jan Novák et al.
Organic letters, 5(5), 637-639 (2003-02-28)
Protecting the hydroxyl group in both 2-bromo-2-phenylethanol and 2-bromo-1-phenylethanol enhanced the alkylation of 2-amino-6-chloropurine to give corresponding 7- and 9-alkylated products. Subsequent hydrolysis and deprotection led to 7- and 9-hydroxy(phenyl)ethylguanines. 7-Hydroxy(phenyl)ethylguanines are major guanine adducts formed by interaction of styrene
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.