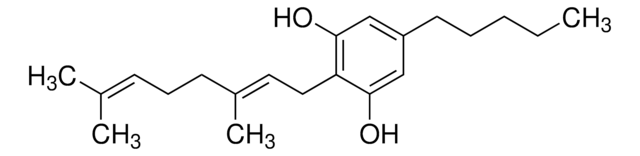
T-032
(−)-Δ8-THC solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produits recommandés
Qualité
certified reference material
Niveau de qualité
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
drug control
Narcotic Licence Schedule D (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IIB (Portugal)
Concentration
1.0 mg/mL in methanol
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
cannabis testing
cannabis testing
Format
single component solution
Température de stockage
−20°C
InChI
1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9,12-13,16-17,22H,5-8,10-11H2,1-4H3
Clé InChI
HCAWPGARWVBULJ-UHFFFAOYSA-N
Description générale
Application
- Multi-analysis of 11 cannabinoids in biomass and extracts of different cannabis varieties by an HPLC-UV method, following the International Conference on Harmonization (ICH) Tripartite Guideline for Validation of Analytical Procedures
- Separation and estimation of 13 cannabinoids from different cannabis products using isocratic high-performance liquid chromatography (HPLC) in combination with a diode array detector (DAD)
- Gas chromatographic-mass spectrometric (GC-MS) analysis of ten main cannabinoids from hemp inflorescences following their silylation and esterification for derivatization
- Quantification of cannabidiol and Δ9-tetrahydrocannabinol in addition to the identification of minor cannabinoids from human plasma samples using ultra-performance liquid chromatography (UHPLC) combined with triple quadrupole mass spectrometry following a liquid-liquid extraction
- QuEChERS based extraction of 15 cannabinoids from 20 food product samples followed by their quantification by liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Caractéristiques et avantages
- Fully characterized under ISO/IEC 17025 and ISO 17034 accreditation
- Accompanied with a comprehensive Certificate of Analysis (CoA) with data on stability, homogeneity, accuracy of concentration, uncertainty, and traceability
- Rigorously tested through real-time stability studies to ensure accuracy and shelf life
- Gravimetrically prepared using qualified precision balances to ensure minimal uncertainty
- Flame sealed under argon into ampoules for long-term shelf life
- Offered in a convenient, DEA-exempt format to improve laboratory efficiency
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
51.8 °F - closed cup
Point d'éclair (°C)
11.0 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
A comprehensive high performance liquid chromatography-diode array detection (HPLC-DAD) workflow for the analysis of 14 cannabinoids in hemp bud extracts within 10 minutes using robust Chromolith® monolithic silica HPLC columns with low column back pressure.
The cannabinoids found in the Cannabis plant commonly referred to as marijuana, have grown in popularity for treating a variety of ailments from arthritis, glaucoma, and chronic pain to malnutrition, multiple sclerosis, and cancer.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material.
Protocoles
Potency testing in marijuana-infused edibles is an important problem that analytical labs are facing due to the complexity of the involved matrices. Concentration of active ingredients in these edibles can range from a few parts per million to 3.5 parts per thousand. This application demonstrates the extraction and HPLC-UV analysis of the active compounds.
As the popularity of cannabis-infused products increases, there is a growing need to characterize the type and content of the cannabinoids found in the product. This application demonstrates the ability of the Ascentis Express C18 column to baseline resolve 14 structurally-similar cannabinoids, in under seven minutes, with excellent peak shape.
Rapid potency testing of marijuana-infused edibles using LC/MS on a biphenyl stationary phase detected eleven cannabinoids.
Tetrahydrocannabinolic acid A solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Cannabichromenic Acid (CBCA) solution, 1.0 mg/mL in acetonitrile, certified reference material, ampule of 1 mL
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique