T7650
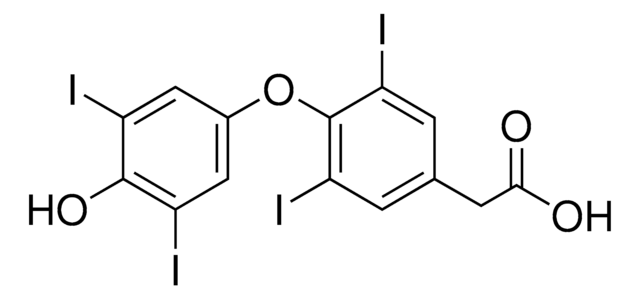
3,3′,5-Triiodothyroacetic acid
≥90%
Synonyme(s) :
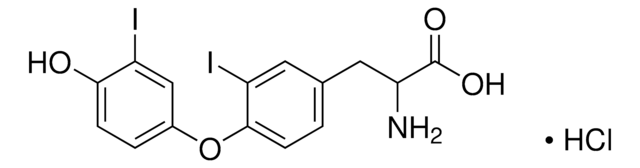
4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenylacetic acid
About This Item
Produits recommandés
Pureté
≥90%
Forme
powder
Température de stockage
−20°C
Chaîne SMILES
OC(=O)Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1
InChI
1S/C14H9I3O4/c15-9-6-8(1-2-12(9)18)21-14-10(16)3-7(4-11(14)17)5-13(19)20/h1-4,6,18H,5H2,(H,19,20)
Clé InChI
UOWZUVNAGUAEQC-UHFFFAOYSA-N
Informations sur le gène
human ... THRA(7067) , THRB(7068)
rat ... Thra(81812) , Thrb(24831)
Application
- Thyroid hormone research: A study explored the impact of 3,3′,5-Triiodothyroacetic acid on peripheral and neurodevelopmental findings in patients with MCT8 deficiency, highlighting its potential in therapeutic interventions for thyroid-related developmental disorders (Unsal and Hayran, 2024).
- Neurological disorder management: Research demonstrated the use of 3,3′,5-Triiodothyroacetic acid in addressing impaired T3 uptake and action in cerebral organoids modeling Allan-Herndon-Dudley syndrome, providing insights into its application in managing brain-specific thyroid hormone transport abnormalities (Salas-Lucia et al., 2024).
- Pharmaceutical development for antiviral therapies: Tiratricol, a derivative of 3,3′,5-Triiodothyroacetic acid, was identified as an inhibitor of yellow fever virus replication by targeting the viral RNA-dependent RNA polymerase, showcasing its potential in antiviral drug development (Ren et al., 2023).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Code de la classe de stockage
13 - Non Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique