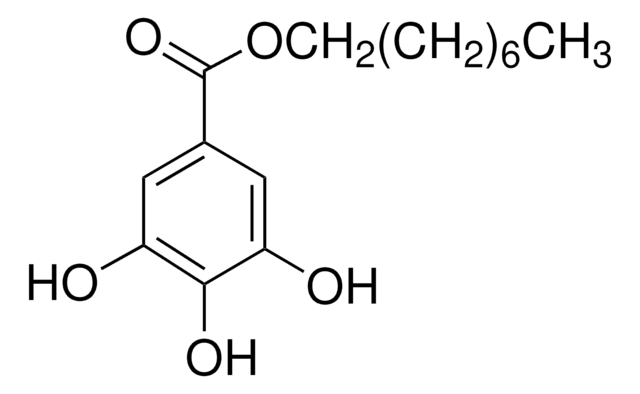
48660
Lauryl gallate
antioxidant, ≥99.0% (HPLC)
Synonyme(s) :
Dodecyl gallate
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥99.0% (HPLC)
Forme
solid
Pf
94-97 °C (lit.)
Groupe fonctionnel
ester
Chaîne SMILES
CCCCCCCCCCCCOC(=O)c1cc(O)c(O)c(O)c1
InChI
1S/C19H30O5/c1-2-3-4-5-6-7-8-9-10-11-12-24-19(23)15-13-16(20)18(22)17(21)14-15/h13-14,20-22H,2-12H2,1H3
Clé InChI
RPWFJAMTCNSJKK-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Properties of artificial phospholipid membranes containing lauryl gallate or cholesterol: This study explores how lauryl gallate affects membrane structure, impacting its biological functions (Jurak et al., 2018).
- Potential of Lauryl Gallate as Stability and Recyclability Improver of Poly (Butylene succinate-co-adipate): Lauryl gallate enhances the stability and recyclability of polyesters, important for sustainable materials (Rossi et al., 2024).
- Lauryl Gallate Activity and Streptococcus mutans: The study evaluates the antibacterial effects of lauryl gallate against Streptococcus mutans, showing significant biofilm reduction and gene expression impact (Gabe et al., 2020).
- Dodecyl gallate as a pro-ecological antioxidant for food packing materials: This research investigates the use of lauryl gallate as a green antioxidant, enhancing the age-resistance of food packaging (Masek et al., 2014).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Skin Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique