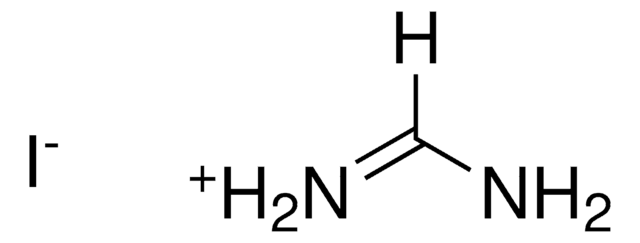
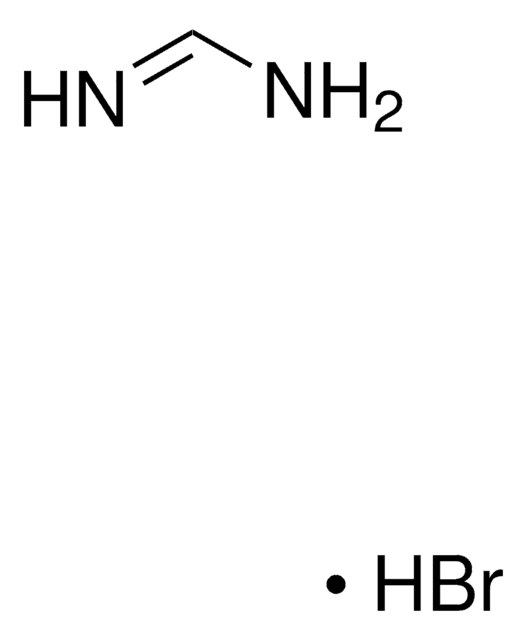
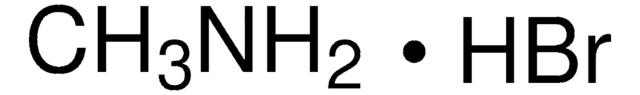398853
Lead(II) bromide
99.999% trace metals basis
Synonyme(s) :
Lead dibromide
About This Item
Produits recommandés
Pureté
99.999% trace metals basis
Forme
powder
Pertinence de la réaction
core: lead
Impuretés
≤15.0 ppm Trace Metal Analysis
Point d'ébullition
892 °C (lit.)
Pf
371 °C (lit.)
Densité
6.66 g/mL at 25 °C (lit.)
Chaîne SMILES
Br[PbH2]Br
InChI
1S/2BrH.Pb/h2*1H;/q;;+2/p-2
Clé InChI
ZASWJUOMEGBQCQ-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- To prepare an electrolyte for a high performance all-solid-state bromide-ion battery.
- As a precursor to prepare organic–inorganic hybrid perovskite materials for solar cells and light emitting devices (LEDs).
- As a starting material to prepare photocatalytic CsPbBr3@SiO2 composites with good water stability.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1A - STOT RE 2
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Since the first report of the low-cost dye-sensitized solar cell (DSSC) in 1991 by Gratzel and his coworker,1 dye-sensitized solar cells (DSSC) has been regarded as one of the most promising photovoltaic technologies because of their transparent and colorful characteristics, as well as low cost.
Colloidal quantum dots (CQDs) are semiconducting crystals of only a few nanometers (ca. 2–12 nm) coated with ligand/surfactant molecules to help prevent agglomeration.
The past several decades have seen major advancements in the synthesis of metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters”.
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique